Oxazolidinones as versatile scaffolds in medicinal chemistry
- PMID: 37252095
- PMCID: PMC10211318
- DOI: 10.1039/d2md00415a
Oxazolidinones as versatile scaffolds in medicinal chemistry
Abstract
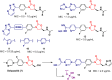
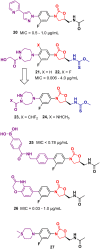
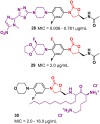
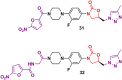
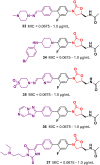
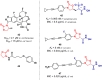
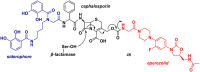
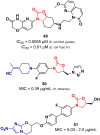
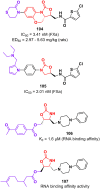
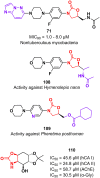
Oxazolidinone is a five-member heterocyclic ring with several biological applications in medicinal chemistry. Among the three possible isomers, 2-oxazolidinone is the most investigated in drug discovery. Linezolid was pioneered as the first approved drug containing an oxazolidinone ring as the pharmacophore group. Numerous analogues have been developed since its arrival on the market in 2000. Some have succeeded in reaching the advanced stages of clinical studies. However, most oxazolidinone derivatives reported in recent decades have not reached the initial stages of drug development, despite their promising pharmacological applications in a variety of therapeutic areas, including antibacterial, antituberculosis, anticancer, anti-inflammatory, neurologic, and metabolic diseases, among other areas. Therefore, this review article aims to compile the efforts of medicinal chemists who have explored this scaffold over the past decades and highlight the potential of the class for medicinal chemistry.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




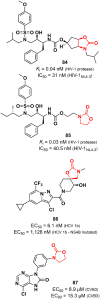
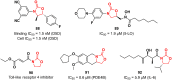
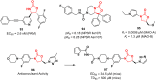
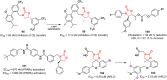
References
-
- Brickner S. J. Curr. Pharm. Des. 1996;2:175–194. doi: 10.2174/1381612802666220921173820. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

