Fornix deepening reconstruction in conjunctivochalasis surgery
- PMID: 37252158
- PMCID: PMC10220437
- DOI: 10.4103/tjo.tjo_28_22
Fornix deepening reconstruction in conjunctivochalasis surgery
Abstract
Purpose: To assess the extent of inferior fornix shortening in conjunctivochalasis (CCh) and to evaluate whether fornix deepening reconstruction can restore the fornix tear reservoir in patients with CCh.
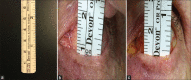
Materials and methods: This was a retrospective review of five patients (3 unilateral and 2 bilateral eyes, total 7 eyes) with CCh who underwent fornix deepening reconstruction with conjunctival recession and amniotic membrane transplantation. Postsurgical outcome measures included changes in fornix depth with correlation to basal tear volumes, symptoms, corneal staining, and conjunctival inflammation.
Results: For the three patients with unilateral surgery, both the fornix depth (8.3 ± 1.5 mm) and wetting length (9.3 ± 8.5 mm) of the operative eyes were less than the fellow eyes (10.3 ± 1.5 mm and 10.3 ± 8.5 mm, respectively). At 5.3 ± 2.7 months (range 1.7-8.7) postoperatively, the fornix depth increased significantly by 2.0 ± 1.1 mm (P = 0.02). Deepening of the fornix depth was accompanied by overwhelming symptomatic relief (91.5%) that could be subdivided into complete relief (87.5%) and partial relief (4%) of symptoms, with blurred vision being the most notably relieved symptom (P = 0.03). Furthermore, superficial punctate keratitis and conjunctival inflammation were significantly improved at follow-up (P = 0.008 and 0.05, respectively).
Conclusion: Deepening of the fornix to restore the tear reservoir is an important surgical objective that may change the tear hydrodynamic state to provide a stable tear film and improve outcomes in CCh.
Keywords: Amniotic membrane; conjunctivochalasis; fornix deepening; fornix reconstruction; tear reservoir.
Copyright: © 2022 Taiwan Journal of Ophthalmology.
Conflict of interest statement
S.T. has obtained a patent for the method of preparation and clinical uses of amniotic membrane and has licensed the rights to TissueTech, Inc., which procures and processes, and to Bio-Tissue, Inc., which is a subsidiary of TissueTech, Inc., to distribute cryopreserved amniotic membrane for clinical and research uses. S.T.O.M. and S.T. are employees of BioTissue, Inc. Dr. Scheffer C.G. Tseng, an editorial board member at Taiwan Journal of Ophthalmology, had no role in the peer review process of or decision to publish this article. The other authors declared no conflicts of interest in writing this paper.
Figures

References
-
- Tseng SC, Tsubota K. Important concepts for treating ocular surface and tear disorders. Am J Ophthalmol. 1997;124:825–35. - PubMed
-
- Meller D, Tseng SC. Conjunctivochalasis: Literature review and possible pathophysiology. Surv Ophthalmol. 1998;43:225–32. - PubMed
-
- Kheirkhah A, Casas V, Blanco G, Li W, Hayashida Y, Chen YT, et al. Amniotic membrane transplantation with fibrin glue for conjunctivochalasis. Am J Ophthalmol. 2007;144:311–3. - PubMed
-
- Liu D. Conjunctivochalasis. A cause of tearing and its management. Ophthalmic Plast Reconstr Surg. 1986;2:25–8. - PubMed
LinkOut - more resources
Full Text Sources
