Free fatty acids support oligodendrocyte survival in a mouse model of amyotrophic lateral sclerosis
- PMID: 37252191
- PMCID: PMC10213402
- DOI: 10.3389/fncel.2023.1081190
Free fatty acids support oligodendrocyte survival in a mouse model of amyotrophic lateral sclerosis
Abstract
Introduction: Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by the white matter degeneration. Although changes in blood lipids are involved in the pathogenesis of neurological diseases, the pathological role of blood lipids in ALS remains unclear.
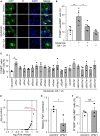
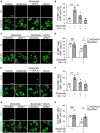
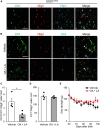
Methods and results: We performed lipidome analysis on the plasma of ALS model mice, mutant superoxide dismutase 1 (SOD1G93A) mice, and found that the concentration of free fatty acids (FFAs), including oleic acid (OA) and linoleic acid (LA), decreased prior to disease onset. An in vitro study revealed that OA and LA directly inhibited glutamate-induced oligodendrocytes cell death via free fatty acid receptor 1 (FFAR1). A cocktail containing OA/LA suppressed oligodendrocyte cell death in the spinal cord of SOD1G93A mice.
Discussion: These results suggested that the reduction of FFAs in the plasma is a pathogenic biomarker for ALS in the early stages, and supplying a deficiency in FFAs is a potential therapeutic approach for ALS by preventing oligodendrocyte cell death.
Keywords: SOD1; amyotrophic lateral sclerosis (ALS); free fatty acids; lipidome; oligodendrocyte.
Copyright © 2023 Maruyama, Tanabe, Uyeda, Suzuki and Muramatsu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Knocking down metabotropic glutamate receptor 1 improves survival and disease progression in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis.Neurobiol Dis. 2014 Apr;64:48-59. doi: 10.1016/j.nbd.2013.11.006. Epub 2013 Dec 19. Neurobiol Dis. 2014. PMID: 24361555
-
In-vivo effects of knocking-down metabotropic glutamate receptor 5 in the SOD1G93A mouse model of amyotrophic lateral sclerosis.Neuropharmacology. 2017 Sep 1;123:433-445. doi: 10.1016/j.neuropharm.2017.06.020. Epub 2017 Jun 21. Neuropharmacology. 2017. PMID: 28645622
-
The Overexpression of TDP-43 Protein in the Neuron and Oligodendrocyte Cells Causes the Progressive Motor Neuron Degeneration in the SOD1 G93A Transgenic Mouse Model of Amyotrophic Lateral Sclerosis.Int J Biol Sci. 2016 Aug 15;12(9):1140-9. doi: 10.7150/ijbs.15938. eCollection 2016. Int J Biol Sci. 2016. PMID: 27570488 Free PMC article.
-
Dysfunction of oligodendrocyte inwardly rectifying potassium channel in a rat model of amyotrophic lateral sclerosis.Eur J Neurosci. 2021 Oct;54(7):6339-6354. doi: 10.1111/ejn.15451. Epub 2021 Sep 22. Eur J Neurosci. 2021. PMID: 34510584
-
Altered expression of atypical PKC and Ryk in the spinal cord of a mouse model of amyotrophic lateral sclerosis.Dev Neurobiol. 2014 Aug;74(8):839-50. doi: 10.1002/dneu.22137. Epub 2014 Jan 22. Dev Neurobiol. 2014. PMID: 24123880 Free PMC article. Review.
Cited by
-
Oligodendrocytes in amyotrophic lateral sclerosis and frontotemporal dementia: the new players on stage.Front Mol Neurosci. 2024 Mar 22;17:1375330. doi: 10.3389/fnmol.2024.1375330. eCollection 2024. Front Mol Neurosci. 2024. PMID: 38585368 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

