Optimizing COVID-19 Vaccination Strategy in Pediatric Kidney Transplant Recipients: Humoral and Cellular Response to SARS-CoV-2 mRNA Vaccination
- PMID: 37252612
- PMCID: PMC10213233
- DOI: 10.3389/ti.2023.11153
Optimizing COVID-19 Vaccination Strategy in Pediatric Kidney Transplant Recipients: Humoral and Cellular Response to SARS-CoV-2 mRNA Vaccination
Abstract
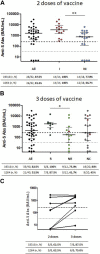
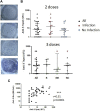
In this retrospective cohort study, we analyze the early humoral and cellular response in 64 adolescents KTx recipients, after two or three doses of mRNA vaccine BNT162b2 against different variants of COVID-19. After 2 doses, 77.8% % of children with no history of infection had a positive humoral response with a median anti-S IgG level of 1107 (IQR, 593-2,658) BAU/mL. All the patients with a history of infection responded with a higher median IgG level (3,265 (IQR, 1,492-8,178) BAU/mL). In non-responders after 2 doses, 75% responded after a third dose with a median Ab titer at 355 (IQR, 140-3,865 BAU/mL). Neutralizing activity was significantly lower against the delta and the omicron variants compared to the wild-type strain and did not improve after a 3rd dose, while infection did provide higher levels of neutralizations against the variants. T cell specific response correlated with humoral response and no patient displayed a cellular response without a humoral response. Adolescent KTx recipients exhibit a high seroconversion rate after only two doses. A third injection, induces a response in the majority of the non-responders patients but did not counterbalance the strong decrease in neutralizing antibody activities against variants highlighting the need for boosters with specific vaccines.
Keywords: COVID-19; children; immunology; kidney transplantation; pediatric.
Copyright © 2023 Nel, Parmentier, Dehoux, Minier, Duneton, Charbit, Baudouin, Bidet, Carol, Cheyssac, Delbet, Guérin-El Khourouj, Louillet, Ulinski, Delaugerre, Carcelain and Hogan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

