Improved isolation of extracellular vesicles by removal of both free proteins and lipoproteins
- PMID: 37252755
- PMCID: PMC10229111
- DOI: 10.7554/eLife.86394
Improved isolation of extracellular vesicles by removal of both free proteins and lipoproteins
Abstract
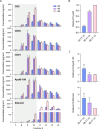
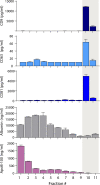
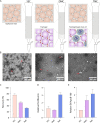
Extracellular vesicles (EVs) are released by all cells into biofluids such as plasma. The separation of EVs from highly abundant free proteins and similarly sized lipoproteins remains technically challenging. We developed a digital ELISA assay based on Single Molecule Array (Simoa) technology for ApoB-100, the protein component of several lipoproteins. Combining this ApoB-100 assay with previously developed Simoa assays for albumin and three tetraspanin proteins found on EVs (Ter-Ovanesyan, Norman et al., 2021), we were able to measure the separation of EVs from both lipoproteins and free proteins. We used these five assays to compare EV separation from lipoproteins using size exclusion chromatography with resins containing different pore sizes. We also developed improved methods for EV isolation based on combining several types of chromatography resins in the same column. We present a simple approach to quantitatively measure the main impurities of EV isolation in plasma and apply this approach to develop novel methods for enriching EVs from human plasma. These methods will enable applications where high-purity EVs are required to both understand EV biology and profile EVs for biomarker discovery.
Keywords: ApoB-100; EVs; biochemistry; chemical biology; exosomes; extracellular vesicles; human; lipoproteins; medicine.
© 2023, Ter-Ovanesyan, Gilboa et al.
Conflict of interest statement
DT DT has filed IP on methods for EV isolation and analysis, TG TG has filed IP on methods for EV isolation and analysis, BB BB has filed IP on methods for EV isolation and analysis, AN, SW, RL, WT No competing interests declared, DK DK has filed IP on methods for EV isolation and analysis, GC GMC has filed IP on methods for EV isolation and analysis, DW DRW has filed IP on methods for EV isolation and analysis
Figures


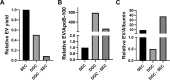




Update of
- doi: 10.1101/2023.01.20.524891
References
-
- Johnsen KB, Gudbergsson JM, Andresen TL, Simonsen JB. What is the blood concentration of extracellular Vesicles? implications for the use of extracellular Vesicles as blood-borne biomarkers of cancer. Biochimica et Biophysica Acta. Reviews on Cancer. 2019;1871:109–116. doi: 10.1016/j.bbcan.2018.11.006. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

