Glial dysregulation in the human brain in fragile X-associated tremor/ataxia syndrome
- PMID: 37252957
- PMCID: PMC10265985
- DOI: 10.1073/pnas.2300052120
Glial dysregulation in the human brain in fragile X-associated tremor/ataxia syndrome
Abstract
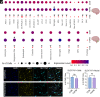
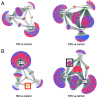
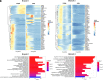
Short trinucleotide expansions at the FMR1 locus are associated with the late-onset condition fragile X-associated tremor/ataxia syndrome (FXTAS), which shows very different clinical and pathological features from fragile X syndrome (associated with longer expansions), with no clear molecular explanation for these marked differences. One prevailing theory posits that the shorter, premutation expansion uniquely causes extreme neurotoxic increases in FMR1 mRNA (i.e., four to eightfold increases), but evidence to support this hypothesis is largely derived from analysis of peripheral blood. We applied single-nucleus RNA sequencing to postmortem frontal cortex and cerebellum from 7 individuals with premutation and matched controls (n = 6) to assess cell type-specific molecular neuropathology. We found only modest upregulation (~1.3-fold) of FMR1 in some glial populations associated with premutation expansions. In premutation cases, we also identified decreased astrocyte proportions in the cortex. Differential expression and gene ontology analysis demonstrated altered neuroregulatory roles of glia. Using network analyses, we identified cell type-specific and region-specific patterns of FMR1 protein target gene dysregulation unique to premutation cases, with notable network dysregulation in the cortical oligodendrocyte lineage. We used pseudotime trajectory analysis to determine how oligodendrocyte development was altered and identified differences in early gene expression in oligodendrocyte trajectories in premutation cases specifically, implicating early cortical glial developmental perturbations. These findings challenge dogma regarding extremely elevated FMR1 increases in FXTAS and implicate glial dysregulation as a critical facet of premutation pathophysiology, representing potential unique therapeutic targets directly derived from the human condition.
Keywords: FMR1; FXTAS; glia; human brain; snRNA-seq.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- Hagerman R. J., “Fragile X syndrome” in Nature Reviews Disease Primers 2017 3:1 (Nature Publishing Group, 2017), pp 1–19.
-
- Pieretti M., Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66, 817–822 (1991). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

