Prognostic evaluation of re-resection for recurrent glioblastoma using the novel RANO classification for extent of resection: A report of the RANO resect group
- PMID: 37253096
- PMCID: PMC10479742
- DOI: 10.1093/neuonc/noad074
Prognostic evaluation of re-resection for recurrent glioblastoma using the novel RANO classification for extent of resection: A report of the RANO resect group
Abstract
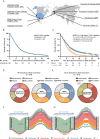
Background: The value of re-resection in recurrent glioblastoma remains controversial as a randomized trial that specifies intentional incomplete resection cannot be justified ethically. Here, we aimed to (1) explore the prognostic role of extent of re-resection using the previously proposed Response Assessment in Neuro-Oncology (RANO) classification (based upon residual contrast-enhancing (CE) and non-CE tumor), and to (2) define factors consolidating the surgical effects on outcome.
Methods: The RANO resect group retrospectively compiled an 8-center cohort of patients with first recurrence from previously resected glioblastomas. The associations of re-resection and other clinical factors with outcome were analyzed. Propensity score-matched analyses were constructed to minimize confounding effects when comparing the different RANO classes.
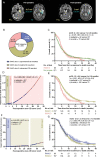
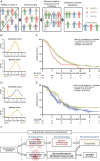
Results: We studied 681 patients with first recurrence of Isocitrate Dehydrogenase (IDH) wild-type glioblastomas, including 310 patients who underwent re-resection. Re-resection was associated with prolonged survival even when stratifying for molecular and clinical confounders on multivariate analysis; ≤1 cm3 residual CE tumor was associated with longer survival than non-surgical management. Accordingly, "maximal resection" (class 2) had superior survival compared to "submaximal resection" (class 3). Administration of (radio-)chemotherapy in the absence of postoperative deficits augmented the survival associations of smaller residual CE tumors. Conversely, "supramaximal resection" of non-CE tumor (class 1) was not associated with prolonged survival but was frequently accompanied by postoperative deficits. The prognostic role of residual CE tumor was confirmed in propensity score analyses.
Conclusions: The RANO resect classification serves to stratify patients with re-resection of glioblastoma. Complete resection according to RANO resect classes 1 and 2 is prognostic.
Keywords: classification; extent of resection; glioblastoma recurrence; outcome; surgical re-resection.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
MW - Research grants: Quercis, Versameb. Honoraria or advisory board participation and consulting: Bayer, CureVac, Medac, Novartis, Orbus, Philogen. M.vdB.
Figures


References
-
- Gorlia T, Stupp R, Brandes AA, et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: A pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur J Cancer. 2012;48(8):1176–1184. - PubMed
-
- Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. - PubMed

