NLRP3 inflammasome and interleukin-1 contributions to COVID-19-associated coagulopathy and immunothrombosis
- PMID: 37253117
- PMCID: PMC10893977
- DOI: 10.1093/cvr/cvad084
NLRP3 inflammasome and interleukin-1 contributions to COVID-19-associated coagulopathy and immunothrombosis
Abstract
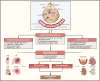
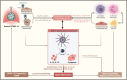
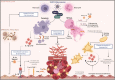
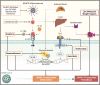
Immunothrombosis-immune-mediated activation of coagulation-is protective against pathogens, but excessive immunothrombosis can result in pathological thrombosis and multiorgan damage, as in severe coronavirus disease 2019 (COVID-19). The NACHT-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome produces major proinflammatory cytokines of the interleukin (IL)-1 family, IL-1β and IL-18, and induces pyroptotic cell death. Activation of the NLRP3 inflammasome pathway also promotes immunothrombotic programs including release of neutrophil extracellular traps and tissue factor by leukocytes, and prothrombotic responses by platelets and the vascular endothelium. NLRP3 inflammasome activation occurs in patients with COVID-19 pneumonia. In preclinical models, NLRP3 inflammasome pathway blockade restrains COVID-19-like hyperinflammation and pathology. Anakinra, recombinant human IL-1 receptor antagonist, showed safety and efficacy and is approved for the treatment of hypoxaemic COVID-19 patients with early signs of hyperinflammation. The non-selective NLRP3 inhibitor colchicine reduced hospitalization and death in a subgroup of COVID-19 outpatients but is not approved for the treatment of COVID-19. Additional COVID-19 trials testing NLRP3 inflammasome pathway blockers are inconclusive or ongoing. We herein outline the contribution of immunothrombosis to COVID-19-associated coagulopathy, and review preclinical and clinical evidence suggesting an engagement of the NLRP3 inflammasome pathway in the immunothrombotic pathogenesis of COVID-19. We also summarize current efforts to target the NLRP3 inflammasome pathway in COVID-19, and discuss challenges, unmet gaps, and the therapeutic potential that inflammasome-targeted strategies may provide for inflammation-driven thrombotic disorders including COVID-19.
Keywords: COVID-19; Coagulation; Endotheliopathy; Interleukin-1; NLRP3 inflammasome; Pyroptosis; Thrombosis.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: N.P. has received a training fellowship from the International Society on Thrombosis and Haemostasis and research funding from the International Network of VENous Thromboembolism Clinical Research Networks (INVENT), outside of the present work. Y.K. is an inventor on a patent application (US20180369278A1) by the University of Michigan on the use of biogases in vascular disease. M.D.N. reports personal fees as an invited speaker from Bayer, Daiichi Sankyo, and Viatris, personal fees for advisory board membership from LEO Pharma and Pfizer, and institutional funding from LEO Pharma. G.K. has received honorary fees from Swedish Orphan Biovitrum, Chugai-Roche, and Amgen. A.B. received a travel grant from Kiniksa Pharmaceuticals Ltd to attend the 2019 AHA Scientific Sessions and honoraria from Effetti s.r.l. (Milan, Italy) to collaborate on the medical website http://www.inflammology.org, outside the present work. J.M.C. has received personal fees for scientific advisory boards and consulting from Abbott, Anthos, Alnylam, Bristol Myers Squibb, Five Prime Therapeutics, Pfizer, Takeda, and research funding from CSL Behring, outside of the submitted work. R.D.C. has received personal fees from Boehringer-Ingelheim, Bayer, BMS-Pfizer, Daiichi Sankyo, Novartis, Roche, Sanofi, Amgen, Milestone, Menarini, AstraZeneca, and Guidotti, outside the submitted work. A.A. has received research grant funding and has served as a paid scientific advisor to Implicit Biosciences, Kiniksa, Lilly, Merck, Novartis, Novo Nordisk, Olatec, R-Pharm, Serpin Pharma, and Swedish Orphan Biovitrum, outside of the submitted work. E.G. has nothing to disclose.
Figures
References
-
- Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res 2016;118:1392–1408. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

