Protocol for a comprehensive prospective cohort study of trio-based whole-genome sequencing for underlying cancer predisposition in paediatric and adolescent patients newly diagnosed with cancer: the PREDICT study
- PMID: 37253493
- PMCID: PMC10254808
- DOI: 10.1136/bmjopen-2022-070082
Protocol for a comprehensive prospective cohort study of trio-based whole-genome sequencing for underlying cancer predisposition in paediatric and adolescent patients newly diagnosed with cancer: the PREDICT study
Abstract
Introduction: Identifying an underlying germline cancer predisposition (CP) in a child with cancer has potentially significant implications for both the child and biological relatives. Cohort studies indicate that 10%-15% of paediatric cancer patients carry germline pathogenic or likely pathogenic variants in cancer predisposition genes, but many of these patients do not meet current clinical criteria for genetic testing. This suggests broad tumour agnostic germline testing may benefit paediatric cancer patients. However, the utility and psychosocial impact of this approach remain unknown. We hypothesise that an approach involving trio whole-genome germline sequencing (trio WGS) will identify children and families with an underlying CP in a timely fashion, that the trio design will streamline cancer risk counselling to at-risk relatives if CP was inherited, and that trio testing will not have a negative psychosocial impact on families.
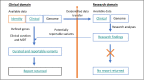
Method and analysis: To test this, we present the Cancer PREDisposition In Childhood by Trio sequencing study (PREDICT). This study will assess the clinical utility of trio WGS to identify CP in unselected patients with cancer 21 years or younger in New South Wales, Australia. PREDICT will perform analysis of biological parents to determine heritability and will examine the psychosocial impact of this trio sequencing approach. PREDICT also includes a broad genomics research programme to identify new candidate genes associated with childhood cancer risk.
Ethics and dissemination: By evaluating the feasibility, utility and psychosocial impact of trio WGS to identify CP in paediatric cancer, PREDICT will inform how such comprehensive testing can be incorporated into a standard of care at diagnosis for all childhood cancer patients.
Trial registration number: NCT04903782.
Keywords: cancer genetics; molecular aspects; molecular biology; paediatric clinical genetics & dysmorphology; paediatric oncology; protocols & guidelines.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
