Antibody blockade of Jagged1 attenuates choroidal neovascularization
- PMID: 37253747
- PMCID: PMC10229650
- DOI: 10.1038/s41467-023-38563-w
Antibody blockade of Jagged1 attenuates choroidal neovascularization
Abstract
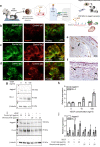
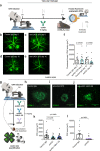
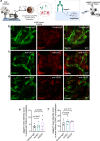
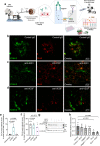
Antibody-based blocking of vascular endothelial growth factor (VEGF) reduces choroidal neovascularization (CNV) and retinal edema, rescuing vision in patients with neovascular age-related macular degeneration (nAMD). However, poor response and resistance to anti-VEGF treatment occurs. We report that targeting the Notch ligand Jagged1 by a monoclonal antibody reduces neovascular lesion size, number of activated phagocytes and inflammatory markers and vascular leakage in an experimental CNV mouse model. Additionally, we demonstrate that Jagged1 is expressed in mouse and human eyes, and that Jagged1 expression is independent of VEGF signaling in human endothelial cells. When anti-Jagged1 was combined with anti-VEGF in mice, the decrease in lesion size exceeded that of either antibody alone. The therapeutic effect was solely dependent on blocking, as engineering antibodies to abolish effector functions did not impair the therapeutic effect. Targeting of Jagged1 alone or in combination with anti-VEGF may thus be an attractive strategy to attenuate CNV-bearing diseases.
© 2023. The Author(s).
Conflict of interest statement
E.S. has received lecture fees from Allergan and consultancy fees from Sanofy Genzyme. M.C.M. has been a member of advisory boards at Bayer, Novartis, Roche and Allergan and has received lecture fees from Bayer and Roche. T.T.G. and J.T.A. have ownership interests in Authera AS. The remaining authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

