Microfibril-associated protein 5 and the regulation of skin scar formation
- PMID: 37253753
- PMCID: PMC10229580
- DOI: 10.1038/s41598-023-35558-x
Microfibril-associated protein 5 and the regulation of skin scar formation
Abstract
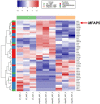
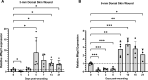
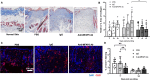
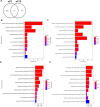
Many factors regulate scar formation, which yields a modified extracellular matrix (ECM). Among ECM components, microfibril-associated proteins have been minimally explored in the context of skin wound repair. Microfibril-associated protein 5 (MFAP5), a small 25 kD serine and threonine rich microfibril-associated protein, influences microfibril function and modulates major extracellular signaling pathways. Though known to be associated with fibrosis and angiogenesis in certain pathologies, MFAP5's role in wound healing is unknown. Using a murine model of skin wound repair, we found that MFAP5 is significantly expressed during the proliferative and remodeling phases of healing. Analysis of existing single-cell RNA-sequencing data from mouse skin wounds identified two fibroblast subpopulations as the main expressors of MFAP5 during wound healing. Furthermore, neutralization of MFAP5 in healing mouse wounds decreased collagen deposition and refined angiogenesis without altering wound closure. In vitro, recombinant MFAP5 significantly enhanced dermal fibroblast migration, collagen contractility, and expression of pro-fibrotic genes. Additionally, TGF-ß1 increased MFAP5 expression and production in dermal fibroblasts. Our findings suggest that MFAP5 regulates fibroblast function and influences scar formation in healing wounds. Our work demonstrates a previously undescribed role for MFAP5 and suggests that microfibril-associated proteins may be significant modulators of wound healing outcomes and scarring.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

