Detection of bornavirus-reactive antibodies and BoDV-1 RNA only in encephalitis patients from virus endemic areas: a comparative serological and molecular sensitivity, specificity, predictive value, and disease duration correlation study
- PMID: 37253816
- PMCID: PMC10228883
- DOI: 10.1007/s15010-023-02048-1
Detection of bornavirus-reactive antibodies and BoDV-1 RNA only in encephalitis patients from virus endemic areas: a comparative serological and molecular sensitivity, specificity, predictive value, and disease duration correlation study
Abstract
Purpose: Human Borna disease virus (BoDV-1) encephalitis is an emerging disease in Germany. This study investigates the spectrum of human BoDV-1 infection, characterizes anti-BoDV-1-antibodies and kinetics, and compares laboratory test performances.
Methods: Three hundred four encephalitis cases, 308 nation-wide neuropsychiatric conditions, 127 well-defined psychiatric cases from Borna disease-endemic areas, and 20 persons with contact to BoDV-1 encephalitis patients or animals were tested for BoDV-1 infections by serology and PCR.
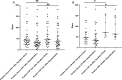
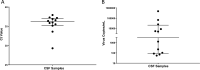
Results: BoDV-1 infections were only found in encephalitis patients with residence in, or recent travel to, virus-endemic areas. Antibodies were detected as early as 12 days after symptom onset. Serum antibody levels correlated with disease duration. Serology was ordered after 50% of the disease duration had elapsed, reflecting low awareness. BoDV-1-antibodies were of IgG1 subclass, and the epitope on BoDV-1 antigens was determined. Specificity of the indirect immunofluorescence antibody test (IFAT) and lineblot (LB) from serum and cerebrospinal fluid (CSF), as well as PCR testing from CSF, was 100%. Sensitivity, depending on first or all samples, reached 75-86% in serum and 92-94% in CSF for the IFAT, and 33-57% in serum and 18-24% in CSF for the LB. Sensitivity for PCR in CSF was 25-67%. Positive predictive values were 100% each, while negative predictive values were 99% (IFAT), 91-97% (LB), and 90% (PCR).
Conclusions: There is no hint that BoDV-1 causes other diseases than encephalitis in humans. Awareness has to be increased in virus-endemic areas. Tests are robust but lack sensitivity. Detection of IgG1 against specific peptides may facilitate diagnosis. Screening of healthy individuals is likely not beneficial.
Keywords: Bornavirus; IgG1; PCR; Peptide; Serology.
© 2023. The Author(s).
Conflict of interest statement
No conflict of interest concerning this publication has to be disclosed by the authors.
Figures





References
-
- Schlottau K, Forth L, Angstwurm K, Höper D, Zecher D, Liesche F, Hoffmann B, Kegel V, Seehofer D, Platen S, Salzberger B, Liebert UG, Niller HH, Schmidt B, Matiasek K, Riemenschneider MJ, Brochhausen C, Banas B, Renders L, Moog P, Wunderlich S, Seifert CL, Barreiros A, Rahmel A, Weiss J, Tappe D, Herden C, Schmidt-Chanasit J, Schwemmle M, Rubbenstroth D, Schlegel J, Pietsch C, Hoffmann D, Jantsch J, Beer M. Fatal encephalitic borna disease virus 1 in solid-organ transplant recipients. N Engl J Med. 2018;379:1377–1379. doi: 10.1056/NEJMc1803115. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

