What About Offering a Financial Incentive Directly to Clinical Units to Encourage the Use of Biosimilars? Results of a Two-Year National Experiment in France
- PMID: 37253898
- PMCID: PMC10228890
- DOI: 10.1007/s40258-023-00812-w
What About Offering a Financial Incentive Directly to Clinical Units to Encourage the Use of Biosimilars? Results of a Two-Year National Experiment in France
Abstract
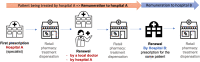
Background: Regarding the low penetration of biosimilars in the French market, in 2018, the government introduced two mutually exclusive financial incentives to increase biosimilar use. They redirect 20% (general case) or 30% (experimental case) of the price difference between the reference product and its biosimilar to hospitals for every biosimilar delivered in retail pharmacies from these hospital prescriptions. The experimental case specifically targets prescribing clinical units.
Objectives: Our study aimed to assess whether the new payment scheme closer to physicians (experimental case) improved etanercept biosimilar penetration after 25 months.
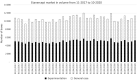
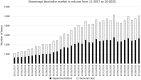
Method: We evaluated hospital prescriptions using IQVIA Xponent data. The monthly number of etanercept boxes delivered in retail pharmacies was linked to the corresponding hospital prescription. The impact of the experimental case on the etanercept biosimilar rate was assessed by a difference-in-difference method.
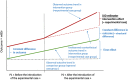
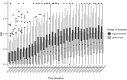
Results: Among the 39 hospitals studied in the experimental case compared with the 169 belonging to the general case, a similar growing trend of etanercept biosimilar use was observed before October 2018. At the start of the experiment, there was an acceleration of biosimilar penetration in the experimental group, until both groups reached a plateau. A significant double difference estimator of 9.72 percentage points in favor of the experiment confirmed this (p < 2.2.10-16).
Conclusion: The French experimental incentive appeared to be more effective at increasing biosimilar use. As it is expected to be implemented in all hospitals, the knowledge gained during this testing phase should allow adjustment of some of its terms and increase physician engagement.
© 2023. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
IQVIA and Biogen France provided us with the dataset for the analysis.
Figures


References
-
- Global medicines use in 2020: outlook and implications. IMS Health for Healthcare Informatics Report, 2015. https://www.imshealth.com/en/thought-leadership/ims-institute/reports/gl.... Accessed 21 Feb 2016.
-
- European Medicines Agency. Biosimilar medicines: Overview [Internet]. 2019 Oct. EMA/627319/2022. https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medici.... Accessed Oct 2022.
-
- IQVIA. The Impact of Biosimilar Competition in Europe 2021. White paper online 2021, Dec. https://www.iqvia.com/library/white-papers/the-impact-of-biosimilar-comp.... Accessed Sept 2022.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

