Identification and localization of polar tube proteins in the extruded polar tube of the microsporidian Anncaliia algerae
- PMID: 37253964
- PMCID: PMC10229552
- DOI: 10.1038/s41598-023-35511-y
Identification and localization of polar tube proteins in the extruded polar tube of the microsporidian Anncaliia algerae
Abstract
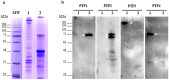
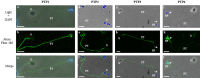
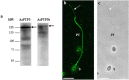
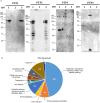
Microsporidia are obligate intracellular parasites able to infect a wide range of hosts from invertebrates to vertebrates. The success of their invasion process is based on an original organelle, the polar tube, which is suddenly extruded from the spore to inoculate the sporoplasm into the host cytoplasm. The polar tube is mainly composed of proteins named polar tube proteins (PTPs). A comparative analysis allowed us to identify genes coding for 5 PTPs (PTP1 to PTP5) in the genome of the microsporidian Anncaliia algerae. While PTP1 and PTP2 are found on the whole polar tube, PTP3 is present in a large part of the extruded polar tube except at its end-terminal part. On the contrary, PTP4 is specifically detected at the end-terminal part of the polar tube. To complete PTPs repertoire, sequential sporal protein extractions were done with high concentration of reducing agents. In addition, a method to purify polar tubes was developed. Mass spectrometry analysis conducted on both samples led to the identification of a PTP3-like protein (PTP3b), and a new PTP (PTP7) only found at the extremity of the polar tube. The specific localization of PTPs asks the question of their roles in cell invasion processes used by A. algerae.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

