The gut microbiome modifies the associations of short- and long-term physical activity with body weight changes
- PMID: 37254152
- PMCID: PMC10228038
- DOI: 10.1186/s40168-023-01542-w
The gut microbiome modifies the associations of short- and long-term physical activity with body weight changes
Abstract
Background: The gut microbiome regulates host energy balance and adiposity-related metabolic consequences, but it remains unknown how the gut microbiome modulates body weight response to physical activity (PA).
Methods: Nested in the Health Professionals Follow-up Study, a subcohort of 307 healthy men (mean[SD] age, 70[4] years) provided stool and blood samples in 2012-2013. Data from cohort long-term follow-ups and from the accelerometer, doubly labeled water, and plasma biomarker measurements during the time of stool collection were used to assess long-term and short-term associations of PA with adiposity. The gut microbiome was profiled by shotgun metagenomics and metatranscriptomics. A subcohort of 209 healthy women from the Nurses' Health Study II was used for validation.
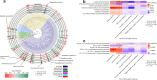
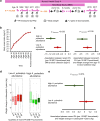
Results: The microbial species Alistipes putredinis was found to modify the association between PA and body weight. Specifically, in individuals with higher abundance of A. putredinis, each 15-MET-hour/week increment in long-term PA was associated with 2.26 kg (95% CI, 1.53-2.98 kg) less weight gain from age 21 to the time of stool collection, whereas those with lower abundance of A. putredinis only had 1.01 kg (95% CI, 0.41-1.61 kg) less weight gain (pinteraction = 0.019). Consistent modification associated with A. putredinis was observed for short-term PA in relation to BMI, fat mass%, plasma HbA1c, and 6-month weight change. This modification effect might be partly attributable to four metabolic pathways encoded by A. putredinis, including folate transformation, fatty acid β-oxidation, gluconeogenesis, and stearate biosynthesis.
Conclusions: A greater abundance of A. putredinis may strengthen the beneficial association of PA with body weight change, suggesting the potential of gut microbial intervention to improve the efficacy of PA in body weight management. Video Abstract.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
- K24 DK098311/NH/NIH HHS/United States
- R01 CA137178/CA/NCI NIH HHS/United States
- R35 CA197735/NH/NIH HHS/United States
- UM1 CA167552/CA/NCI NIH HHS/United States
- R01 AG077489/AG/NIA NIH HHS/United States
- R00 CA215314/NH/NIH HHS/United States
- P01 CA087969/CA/NCI NIH HHS/United States
- R00 CA215314/CA/NCI NIH HHS/United States
- UM1 CA186107/CA/NCI NIH HHS/United States
- P01 CA055075/CA/NCI NIH HHS/United States
- R00 DK119412/DK/NIDDK NIH HHS/United States
- R01 CA202704/CA/NCI NIH HHS/United States
- U01 CA176726/CA/NCI NIH HHS/United States
- U01 CA167552/CA/NCI NIH HHS/United States
- R01 CA151993/CA/NCI NIH HHS/United States
- U01 CA261961/CA/NCI NIH HHS/United States
- R01 CA263776/CA/NCI NIH HHS/United States
- K99 CA283146/CA/NCI NIH HHS/United States
- R01 NR019992/NR/NINR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

