Breast metastatic tumors in lung can be substituted by lung-derived malignant cells transformed by alternative splicing H19 lncRNA
- PMID: 37254190
- PMCID: PMC10228081
- DOI: 10.1186/s13058-023-01662-z
Breast metastatic tumors in lung can be substituted by lung-derived malignant cells transformed by alternative splicing H19 lncRNA
Abstract
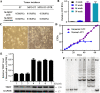
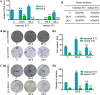
Metastasis accounts for most cancer-associated deaths; yet, this complex process remains poorly understood, particularly the relationship between distant metastasis and primary site-derived cells. Here, we modified the classical MMTV-PyMT breast carcinoma model to trace the fate of mammary-derived carcinoma cells. We show that within the lung, when the metastatic breast carcinoma cells are conditionally depleted, transformed lung epithelial cells generate new metastases. Metastatic breast carcinoma cells transmit H19 long noncoding (lnc) RNA to lung epithelial cells through exosomes. SF3B1 bearing mutations at arginine-625 alternatively splices H19 lncRNA in lung epithelial cells, which selectively acts like a molecular sponge to sequester let-7a and induces Myc upregulation. Under the conditional elimination of primary site-derived breast carcinoma cells, lung malignant cells expressing the mutated SF3B1 splice variant dominate the newly created tumors. Our study suggests that these new carcinoma cells originating from within the colonized organ can replace the primary site-derived malignant cells whenever their expansion is abrogated using an inducible diphtheria toxin receptor in our designed system. These findings should call for a better understanding of metastatic tumors with the specific origin during cancer metastasis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

