Imaging in metastatic breast cancer, CT, PET/CT, MRI, WB-DWI, CCA: review and new perspectives
- PMID: 37254225
- PMCID: PMC10230813
- DOI: 10.1186/s40644-023-00557-8
Imaging in metastatic breast cancer, CT, PET/CT, MRI, WB-DWI, CCA: review and new perspectives
Abstract
Background: Breast cancer is the most frequent cancer in women and remains the second leading cause of death in Western countries. It represents a heterogeneous group of diseases with diverse tumoral behaviour, treatment responsiveness and prognosis. While major progress in diagnosis and treatment has resulted in a decline in breast cancer-related mortality, some patients will relapse and prognosis in this cohort of patients remains poor. Treatment is determined according to tumor subtype; primarily hormone receptor status and HER2 expression. Menopausal status and site of disease relapse are also important considerations in treatment protocols.
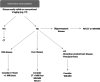
Main body: Staging and repeated evaluation of patients with metastatic breast cancer are central to the accurate assessment of disease extent at diagnosis and during treatment; guiding ongoing clinical management. Advances have been made in the diagnostic and therapeutic fields, particularly with new targeted therapies. In parallel, oncological imaging has evolved exponentially with the development of functional and anatomical imaging techniques. Consistent, reproducible and validated methods of assessing response to therapy is critical in effectively managing patients with metastatic breast cancer.
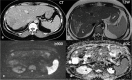
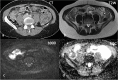
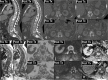
Conclusion: Major progress has been made in oncological imaging over the last few decades. Accurate disease assessment at diagnosis and during treatment is important in the management of metastatic breast cancer. CT (and BS if appropriate) is generally widely available, relatively cheap and sufficient in many cases. However, several additional imaging modalities are emerging and can be used as adjuncts, particularly in pregnancy or other diagnostically challenging cases. Nevertheless, no single imaging technique is without limitation. The authors have evaluated the vast array of imaging techniques - individual, combined parametric and multimodal - that are available or that are emerging in the management of metastatic breast cancer. This includes WB DW-MRI, CCA, novel PET breast cancer-epitope specific radiotracers and radiogenomics.
Keywords: Anatomo-functional imaging; Metastatic breast cancer; Multimodal; Multiparametric; Response assessment; Staging.
© 2023. Crown.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

