Notch3/Hes5 Induces Vascular Dysfunction in Hypoxia-Induced Pulmonary Hypertension Through ER Stress and Redox-Sensitive Pathways
- PMID: 37254738
- PMCID: PMC10355806
- DOI: 10.1161/HYPERTENSIONAHA.122.20449
Notch3/Hes5 Induces Vascular Dysfunction in Hypoxia-Induced Pulmonary Hypertension Through ER Stress and Redox-Sensitive Pathways
Abstract
Background: Notch3 (neurogenic locus notch homolog protein 3) is implicated in vascular diseases, including pulmonary hypertension (PH)/pulmonary arterial hypertension. However, molecular mechanisms remain elusive. We hypothesized increased Notch3 activation induces oxidative and endoplasmic reticulum (ER) stress and downstream redox signaling, associated with procontractile pulmonary artery state, pulmonary vascular dysfunction, and PH development.
Methods: Studies were performed in TgNotch3R169C mice (harboring gain-of-function [GOF] Notch3 mutation) exposed to chronic hypoxia to induce PH, and examined by hemodynamics. Molecular and cellular studies were performed in pulmonary artery smooth muscle cells from pulmonary arterial hypertension patients and in mouse lung. Notch3-regulated genes/proteins, ER stress, ROCK (Rho-associated kinase) expression/activity, Ca2+ transients and generation of reactive oxygen species, and nitric oxide were measured. Pulmonary vascular reactivity was assessed in the presence of fasudil (ROCK inhibitor) and 4-phenylbutyric acid (ER stress inhibitor).
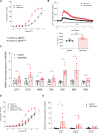
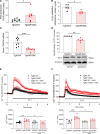
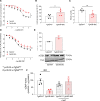
Results: Hypoxia induced a more severe PH phenotype in TgNotch3R169C mice versus controls. TgNotch3R169C mice exhibited enhanced Notch3 activation and expression of Notch3 targets Hes Family BHLH Transcription Factor 5 (Hes5), with increased vascular contraction and impaired vasorelaxation that improved with fasudil/4-phenylbutyric acid. Notch3 mutation was associated with increased pulmonary vessel Ca2+ transients, ROCK activation, ER stress, and increased reactive oxygen species generation, with reduced NO generation and blunted sGC (soluble guanylyl cyclase)/cGMP signaling. These effects were ameliorated by N-acetylcysteine. pulmonary artery smooth muscle cells from patients with pulmonary arterial hypertension recapitulated Notch3/Hes5 signaling, ER stress and redox changes observed in PH mice.
Conclusions: Notch3 GOF amplifies vascular dysfunction in hypoxic PH. This involves oxidative and ER stress, and ROCK. We highlight a novel role for Notch3/Hes5-redox signaling and important interplay between ER and oxidative stress in PH.
Keywords: calcium signaling; endoplasmic reticulum; hypoxia; pulmonary hypertension; reactive oxygen species.
Conflict of interest statement
Figures



References
-
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770 - PubMed
-
- Coupland K, Lendahl U, Karlström H. Role of NOTCH3 mutations in the cerebral small vessel disease cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2018;49:2793–2800. doi: 10.1161/STROKEAHA.118.021560 - PubMed
-
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, et al. . Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

