Pancreatic tumors exhibit myeloid-driven amino acid stress and upregulate arginine biosynthesis
- PMID: 37254839
- PMCID: PMC10260022
- DOI: 10.7554/eLife.81289
Pancreatic tumors exhibit myeloid-driven amino acid stress and upregulate arginine biosynthesis
Abstract
Nutrient stress in the tumor microenvironment requires cancer cells to adopt adaptive metabolic programs for survival and proliferation. Therefore, knowledge of microenvironmental nutrient levels and how cancer cells cope with such nutrition is critical to understand the metabolism underpinning cancer cell biology. Previously, we performed quantitative metabolomics of the interstitial fluid (the local perfusate) of murine pancreatic ductal adenocarcinoma (PDAC) tumors to comprehensively characterize nutrient availability in the microenvironment of these tumors. Here, we develop
Keywords: amino acid homeostasis; arginine; biochemistry; cancer biology; chemical biology; human; metabolism; mouse; nutrient stress; tumor microenvironment.
© 2023, Apiz Saab, Dzierozynski et al.
Conflict of interest statement
JA, LD, PJ, RA, HS, RM, AS, ZN, ZZ, RC, MO, CS, DW, MP, CW, AM No competing interests declared, CL has received consulting fees from Astellas Pharmaceuticals, Odyssey Therapeutics, and T-Knife Therapeutics, and is an inventor on patents pertaining to Kras regulated metabolic pathways, redox control pathways in pancreatic cancer, and targeting the GOT1-pathway as a therapeutic approach (US Patent No: 2015126580-A1, 05/07/2015; US Patent No: 20190136238, 05/09/2019; International Patent No: WO2013177426-A2, 04/23/2015), KM Reviewing editor, eLife
Figures








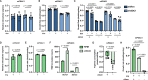



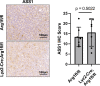

Comment in
-
How nearby nutrients shape tumor growth.Elife. 2023 Jul 17;12:e89825. doi: 10.7554/eLife.89825. Elife. 2023. PMID: 37459171 Free PMC article.
References
-
- Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Grüning BA, Guerler A, Hillman-Jackson J, Hiltemann S, Jalili V, Rasche H, Soranzo N, Goecks J, Taylor J, Nekrutenko A, Blankenberg D. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Research. 2018;46:W537–W544. doi: 10.1093/nar/gky379. - DOI - PMC - PubMed
-
- Auciello FR, Bulusu V, Oon C, Tait-Mulder J, Berry M, Bhattacharyya S, Tumanov S, Allen-Petersen BL, Link J, Kendsersky ND, Vringer E, Schug M, Novo D, Hwang RF, Evans RM, Nixon C, Dorrell C, Morton JP, Norman JC, Sears RC, Kamphorst JJ, Sherman MH. A Stromal Lysolipid-Autotaxin signaling axis promotes Pancreatic tumor progression. Cancer Discovery. 2019;9:617–627. doi: 10.1158/2159-8290.CD-18-1212. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99 CA267176/CA/NCI NIH HHS/United States
- F31 CA257533/CA/NCI NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- R37 CA258346/CA/NCI NIH HHS/United States
- P30 CA014599/CA/NCI NIH HHS/United States
- F32 CA260735/CA/NCI NIH HHS/United States
- T32 CA009594/CA/NCI NIH HHS/United States
- R01 NS129123/NS/NINDS NIH HHS/United States
- UL1 TR002389/TR/NCATS NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- K08 CA234416/CA/NCI NIH HHS/United States
- U2C DK110768/DK/NIDDK NIH HHS/United States
- T32 GM007315/GM/NIGMS NIH HHS/United States
- P30 DK089503/DK/NIDDK NIH HHS/United States
- T32 HD007505/HD/NICHD NIH HHS/United States
- R25 GM143298/GM/NIGMS NIH HHS/United States
- P60 DK020572/DK/NIDDK NIH HHS/United States
- R25 GM086262/GM/NIGMS NIH HHS/United States
- R01 CA200310/CA/NCI NIH HHS/United States
- R37 CA237421/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

