Human embryo implantation
- PMID: 37254877
- PMCID: PMC10281521
- DOI: 10.1242/dev.201507
Human embryo implantation
Abstract
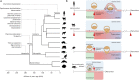
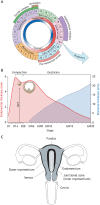
Embryo implantation in humans is interstitial, meaning the entire conceptus embeds in the endometrium before the placental trophoblast invades beyond the uterine mucosa into the underlying inner myometrium. Once implanted, embryo survival pivots on the transformation of the endometrium into an anti-inflammatory placental bed, termed decidua, under homeostatic control of uterine natural killer cells. Here, we examine the evolutionary context of embryo implantation and elaborate on uterine remodelling before and after conception in humans. We also discuss the interactions between the embryo and the decidualising endometrium that regulate interstitial implantation and determine embryo fitness. Together, this Review highlights the precarious but adaptable nature of the implantation process.
Keywords: Aneuploidy; Decidualisation; Implantation; Pregnancy; Uterine remodelling.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
-
- Aberkane, A., Essahib, W., Spits, C., De Paepe, C., Sermon, K., Adriaenssens, T., Mackens, S., Tournaye, H., Brosens, J. J. and Van De Velde, H. (2018). Expression of adhesion and extracellular matrix genes in human blastocysts upon attachment in a 2D co-culture system. Mol. Hum. Reprod. 24, 375-387. 10.1093/molehr/gay024 - DOI - PubMed
-
- Al-Sabbagh, M., Fusi, L., Higham, J., Lee, Y., Lei, K., Hanyaloglu, A. C., Lam, E. W., Christian, M. and Brosens, J. J. (2011). NADPH oxidase-derived reactive oxygen species mediate decidualization of human endometrial stromal cells in response to cyclic AMP signaling. Endocrinology 152, 730-740. 10.1210/en.2010-0899 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

