Microbiota transfer following liver surgery involves microbial extracellular vesicle migration that affects liver immunity
- PMID: 37255349
- PMCID: PMC10234462
- DOI: 10.1097/HC9.0000000000000164
Microbiota transfer following liver surgery involves microbial extracellular vesicle migration that affects liver immunity
Abstract
Background: Short-term perioperative administration of probiotics was shown to alleviate postoperative complications and promote liver recovery among patients undergoing resection for liver malignancy. The mechanisms by which probiotic bacteria effectively influence the gut microbiome composition during the perioperative time are controversial. Here, we aim to elucidate the short-term direct biological effect of probiotic microbiota-derived vesicles on host liver cells during the perioperative period.
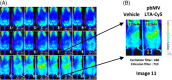
Methods: Probiotic-derived vesicles (pbMVs) were administered postoperatively. pbMVs were isolated and characterized from probiotics, mainly from the bacteria genus Lactobacillus, Bifidobacterium, and Lactococcus. Mice underwent bile duct ligation, sham laparotomy (SHAM), or 70% partial hepatectomy (70%PH). pbMVs were tracked in vivo, and intrahepatic cellular and molecular aspects were analyzed by flow cytometry and qRT-PCR techniques. Liver sinusoidal endothelial cells (LSECs) analysis for Vascular Cell Adhesion Molecule-1(VCAM-1) expression following pbMV stimulation of cultured liver non-parenchymal cells which had been activated by LPS.
Results: The administered pbMV rapidly translocated to the liver after surgery. pbMV administrations following surgeries enhanced neutrophil clearance; there was a dramatic decline in the liver neutrophil-to-lymphocyte ratio Ly6G+/CD3+ and an increase in IL6 levels. pbMVs reduced intrahepatic VCAM1 and ICAM2 expression compared with control following SHAM and decrease in IL10 levels following 70%PH. The administration of pbMV improved liver regeneration 72 hours following surgical liver resection with a significant decrease in IL17 expression. pbMVs modulated VCAM-1 on liver sinusoidal endothelial cells in liver cell culture.
Conclusions: Our study findings provide mechanistic insights into the liver-gut axis following surgery and illustrate how probiotic vesicles can reduce adhesion molecule expression and affect immune cell invasion and liver immunity, resulting in improved liver recovery following hepatic surgery.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Peralta C, Jimenez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol. 2013;59:1094–106. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

