Pediatric Swine Model of Methicillin-Resistant Staphylococcus aureus Sepsis-Induced Coagulopathy, Disseminated Microvascular Thrombosis, and Organ Injuries
- PMID: 37255626
- PMCID: PMC10226618
- DOI: 10.1097/CCE.0000000000000916
Pediatric Swine Model of Methicillin-Resistant Staphylococcus aureus Sepsis-Induced Coagulopathy, Disseminated Microvascular Thrombosis, and Organ Injuries
Abstract
Sepsis-induced coagulopathy leading to disseminated microvascular thrombosis is associated with high mortality and has no existing therapy. Despite the high prevalence of Gram-positive bacterial sepsis, especially methicillin-resistant Staphylococcus aureus (MRSA), there is a paucity of published Gram-positive pediatric sepsis models. Large animal models replicating sepsis-induced coagulopathy are needed to test new therapeutics before human clinical trials.
Hypothesis: Our objective is to develop a pediatric sepsis-induced coagulopathy swine model that last 70 hours.
Methods and models: Ten 3 weeks old piglets, implanted with telemetry devices for continuous hemodynamic monitoring, were IV injected with MRSA (n = 6) (USA300, Texas Children's Hospital 1516 strain) at 1 × 109 colony forming units/kg or saline (n = 4). Fluid resuscitation was given for heart rate greater than 50% or mean arterial blood pressure less than 30% from baseline. Acetaminophen and dextrose were provided as indicated. Point-of-care complete blood count, prothrombin time (PT), activated thromboplastin time, d-dimer, fibrinogen, and specialized coagulation assays were performed at pre- and post-injection, at 0, 24, 48, 60, and 70 hours. Piglets were euthanized and necropsies performed.
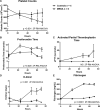
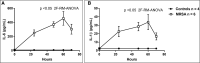
Results: Compared with the saline treated piglets (control), the septic piglets within 24 hours had significantly lower neurologic and respiratory scores. Over time, PT, d-dimer, and fibrinogen increased, while platelet counts and activities of factors V, VII, protein C, antithrombin, and a disintegrin and metalloproteinase with thrombospondin-1 motifs (13th member of the family) (ADAMTS-13) decreased significantly in septic piglets compared with control. Histopathologic examination showed minor focal organ injuries including microvascular thrombi and necrosis in the kidney and liver of septic piglets.
Interpretations and conclusions: We established a 70-hour swine model of MRSA sepsis-induced coagulopathy with signs of consumptive coagulopathy, disseminated microvascular thrombosis, and early organ injuries with histological minor focal organ injuries. This model is clinically relevant to pediatric sepsis and can be used to study dysregulated host immune response and coagulopathy to infection, identify potential early biomarkers, and to test new therapeutics.
Keywords: disseminated; intravascular coagulation; methicillin-resistant Staphylococcus aureus; organ injury; pediatrics; sepsis; swine.
Copyright © 2023 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Conflict of interest statement
Drs. Nguyen, Vijayan, and Cruz received funding from National Institute of General Medical Sciences (NIGMS; R01GM112806). Dr. Marini received funding from NIGMS (R01GM108940). The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures



References
-
- Hershey TB, Kahn JM: State sepsis mandates - a new era for regulation of hospital quality. N Engl J Med 2017; 376:2311–2313 - PubMed
-
- Centers for Disease Control and Prevention: CDC Urges Early Recognition, Prompt Treatment of Sepsis. Centers for Disease Control and Prevention 2017 Report. 2017. Available at: https://www.cdc.gov/media/releases/2017/p0831-sepsis-recognition-treatme... Accessed May 8, 2023
-
- Weiner LM, Webb AK, Limbago B, et al. : Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011-2014. Infect Control Hosp Epidemiol 2016; 37:1288–1301 - PMC - PubMed
-
- Vincent JL, Rello J, Marshall J, et al. : International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009; 302:2323–2329 - PubMed
-
- Laupland KB: Incidence of bloodstream infection: A review of population-based studies. Clin Microbiol Infect 2013; 19:492–500 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

