Ten-year experience with pharmacogenetic testing for DPYD in a national cancer center in Italy: Lessons learned on the path to implementation
- PMID: 37256229
- PMCID: PMC10225682
- DOI: 10.3389/fphar.2023.1199462
Ten-year experience with pharmacogenetic testing for DPYD in a national cancer center in Italy: Lessons learned on the path to implementation
Abstract
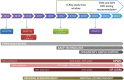
Background: Awareness about the importance of implementing DPYD pharmacogenetics in clinical practice to prevent severe side effects related to the use of fluoropyrimidines has been raised over the years. Since 2012 at the National Cancer Institute, CRO-Aviano (Italy), a diagnostic DPYD genotyping service was set up. Purpose: This study aims to describe the evolution of DPYD diagnostic activity at our center over the last 10 years as a case example of a successful introduction of pharmacogenetic testing in clinical practice. Methods: Data related to the diagnostic activity of in-and out-patients referred to our service between January 2012 and December 2022 were retrieved from the hospital database. Results: DPYD diagnostic activity at our center has greatly evolved over the years, shifting gradually from a post-toxicity to a pre-treatment approach. Development of pharmacogenetic guidelines by national and international consortia, genotyping, and IT technology evolution have impacted DPYD testing uptake in the clinics. Our participation in a large prospective implementation study (Ubiquitous Pharmacogenomics) increased health practitioners' and patients' awareness of pharmacogenetic matters and provided additional standardized infrastructures for genotyping and reporting. Nationwide test reimbursement together with recommendations by regulatory agencies in Europe and Italy in 2020 definitely changed the clinical practice guidelines of fluoropyrimidines prescription. A dramatic increase in the number of pre-treatment DPYD genotyping and in the coverage of new fluoropyrimidine prescriptions was noticed by the last year of observation (2022). Conclusion: The long path to a successful DPYD testing implementation in the clinical practice of a National Cancer Center in Italy demonstrated that the development of pharmacogenetic guidelines and genotyping infrastructure standardization as well as capillary training and education activity for all the potential stakeholders are fundamental. However, only national health politics of test reimbursement and clear recommendations by drug regulatory agencies will definitely move the field forward.
Keywords: CDSS; DPYD; genotyping; implementation; pharmacogenetics; phenotyping.
Copyright © 2023 Bignucolo, De Mattia, Roncato, Peruzzi, Scarabel, D’Andrea, Sartor, Toffoli and Cecchin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- aifa.gov (2020). Nota AIFA. Avialble at: https://www.aifa.gov.it/ .
-
- Amstutz U., Henricks L. M., Offer S. M., Barbarino J., Schellens J. H. M., Swen J. J., et al. (2018). Clinical pharmacogenetics implementation consortium (CPIC) guideline for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing: 2017 update. Clin. Pharmacol. Ther. 103, 210–216. 10.1002/cpt.911 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

