Percutaneous Coronary Intervention vs Coronary Artery Bypass Graft Surgery for Left Main Disease in Patients With and Without Acute Coronary Syndromes: A Pooled Analysis of 4 Randomized Clinical Trials
- PMID: 37256598
- PMCID: PMC10233454
- DOI: 10.1001/jamacardio.2023.1177
Percutaneous Coronary Intervention vs Coronary Artery Bypass Graft Surgery for Left Main Disease in Patients With and Without Acute Coronary Syndromes: A Pooled Analysis of 4 Randomized Clinical Trials
Abstract
Importance: Patients with left main coronary artery disease presenting with an acute coronary syndrome (ACS) represent a high-risk and understudied subgroup of patients with atherosclerosis.
Objective: To assess clinical outcomes after PCI vs CABG in patients with left main disease with vs without ACS.
Design, setting, and participants: Data were pooled from 4 trials comparing PCI with drug-eluting stents vs CABG in patients with left main disease who were considered equally suitable candidates for either strategy (SYNTAX, PRECOMBAT, NOBLE, and EXCEL). Patients were categorized as presenting with or without ACS. Kaplan-Meier event rates through 5 years and Cox model hazard ratios were generated, and interactions were tested. Patients were enrolled in the individual trials from 2004 through 2015. Individual patient data from the trials were pooled and reconciled from 2020 to 2021, and the analyses pertaining to the ACS subgroup were performed from March 2022 through February 2023.
Main outcomes and measures: The primary outcome was death through 5 years. Secondary outcomes included cardiovascular death, spontaneous myocardial infarction (MI), procedural MI, stroke, and repeat revascularization.
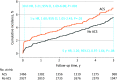
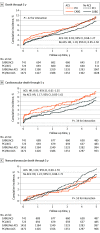
Results: Among 4394 patients (median [IQR] age, 66 [59-73] years; 3371 [76.7%] male and 1022 [23.3%] female) randomized to receive PCI or CABG, 1466 (33%) had ACS. Patients with ACS were more likely to have diabetes, prior MI, left ventricular ejection fraction less than 50%, and higher SYNTAX scores. At 30 days, patients with ACS had higher all-cause death (hazard ratio [HR], 3.40; 95% CI, 1.81-6.37; P < .001) and cardiovascular death (HR, 3.21; 95% CI, 1.69-6.08; P < .001) compared with those without ACS. Patients with ACS also had higher rates of spontaneous MI (HR, 1.70; 95% CI, 1.25-2.31; P < .001) through 5 years. The rates of all-cause mortality through 5 years with PCI vs CABG were 10.9% vs 11.5% (HR, 0.93; 95% CI, 0.68-1.27) in patients with ACS and 11.3% vs 9.6% (HR, 1.19; 95% CI, 0.95-1.50) in patients without ACS (P = .22 for interaction). The risk of early stroke was lower with PCI vs CABG (ACS: HR, 0.39; 95% CI, 0.12-1.25; no ACS: HR, 0.35; 95% CI, 0.16-0.75), whereas the 5-year risks of spontaneous MI and repeat revascularization were higher with PCI vs CABG (spontaneous MI: ACS: HR, 1.74; 95% CI, 1.09-2.77; no ACS: HR, 3.03; 95% CI, 1.94-4.72; repeat revascularization: ACS: HR, 1.57; 95% CI, 1.19-2.09; no ACS: HR, 1.90; 95% CI, 1.54-2.33), regardless of ACS status.
Conclusion and relevance: Among largely stable patients undergoing left main revascularization and with predominantly low to intermediate coronary anatomical complexity, those with ACS had higher rates of early death. Nonetheless, rates of all-cause mortality through 5 years were similar with PCI vs CABG in this high-risk subgroup. The relative advantages and disadvantages of PCI vs CABG in terms of early stroke and long-term spontaneous MI and repeat revascularization were consistent regardless of ACS status.
Trial registration: ClinicalTrials.gov Identifiers: NCT00114972, NCT00422968, NCT01496651, NCT01205776.
Conflict of interest statement
Figures
References
-
- Sabatine MS, Bergmark BA, Murphy SA, et al. Percutaneous coronary intervention with drug-eluting stents versus coronary artery bypass grafting in left main coronary artery disease: an individual patient data meta-analysis. Lancet. 2021;398(10318):2247-2257. doi: 10.1016/S0140-6736(21)02334-5 - DOI - PubMed
-
- Sipahi I, Akay MH, Dagdelen S, Blitz A, Alhan C. Coronary artery bypass grafting vs percutaneous coronary intervention and long-term mortality and morbidity in multivessel disease: meta-analysis of randomized clinical trials of the arterial grafting and stenting era. JAMA Intern Med. 2014;174(2):223-230. doi: 10.1001/jamainternmed.2013.12844 - DOI - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

