Development of a Skin-Directed Scoring System for Stevens-Johnson Syndrome and Epidermal Necrolysis: A Delphi Consensus Exercise
- PMID: 37256599
- PMCID: PMC10838134
- DOI: 10.1001/jamadermatol.2023.1347
Development of a Skin-Directed Scoring System for Stevens-Johnson Syndrome and Epidermal Necrolysis: A Delphi Consensus Exercise
Erratum in
-
Error in Author Surname in Byline.JAMA Dermatol. 2023 Jul 1;159(7):793. doi: 10.1001/jamadermatol.2023.2502. JAMA Dermatol. 2023. PMID: 37466714 Free PMC article. No abstract available.
Abstract
Importance: Scoring systems for Stevens-Johnson syndrome and epidermal necrolysis (EN) only estimate patient prognosis and are weighted toward comorbidities and systemic features; morphologic terminology for EN lesions is inconsistent.
Objectives: To establish consensus among expert dermatologists on EN terminology, morphologic progression, and most-affected sites, and to build a framework for developing a skin-directed scoring system for EN.
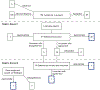
Evidence review: A Delphi consensus using the RAND/UCLA appropriateness criteria was initiated with a core group from the Society of Dermatology Hospitalists to establish agreement on the optimal design for an EN cutaneous scoring instrument, terminology, morphologic traits, and sites of involvement.
Findings: In round 1, the 54 participating dermatology hospitalists reached consensus on all 49 statements (30 appropriate, 3 inappropriate, 16 uncertain). In round 2, they agreed on another 15 statements (8 appropriate, 7 uncertain). There was consistent agreement on the need for a skin-specific instrument; on the most-often affected skin sites (head and neck, chest, upper back, ocular mucosa, oral mucosa); and that blanching erythema, dusky erythema, targetoid erythema, vesicles/bullae, desquamation, and erosions comprise the morphologic traits of EN and can be consistently differentiated.
Conclusions and relevance: This consensus exercise confirmed the need for an EN skin-directed scoring system, nomenclature, and differentiation of specific morphologic traits, and identified the sites most affected. It also established a baseline consensus for a standardized EN instrument with consistent terminology.
Conflict of interest statement
A.G.O.L. has served in advisory boards for Janssen, BMS, and Boeheringer Ingelheim, and as a consultant for Genentech and Guidepoint. He has also received research grants from Eli Lilly Co, OHSU SOM Gerlinger research award, and Medical Research Foundation of Oregon.
L.S.V. currently is an employee and shareholder of Eli Lily and Company. L.S.V had advisory board relationships with Novartis, Boehringer Ingelheim, Helsinn, Kyowa Kirin, Regeneron, Blueprint. She or her institution received research funding from Eli Lilly and Company, Soligenix, Helsinn, Eisai, Boehringer Ingelheim, Novartis, AbbVie, BMS, Celgene, Glenmark, Kyowa Kirin, Amgen, AnaptysBio, Innate Pharma. L.S.V. has been a speaker for Helsinn, Kyowa Kirin.
A.M. serves as a consultant for Alira Health Ventures and Blueprint Medicines. She has received research funding from Incyte Corp and Amryt Pharma and royalties from UpToDate.
B.E.S. is an Assistant Section Editor for JAMA Dermatology.
S.T.C. serves as a consultant for Pfizer, Novartis, Scholar Rock, and VisualDx. He has received honoraria from Pfizer for work on an advisory board for digital media.
M.R. is a consultant for Merck, Janssen, Novartis, Processa, and Eli Lilly. He receives research funding from Processa.
All other authors declare that they have no relevant conflicts of interest.
Figures
References
-
- Lee HY, Walsh SA, Creamer D . Long-term complications of Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow-up. Br J Dermatol. 2017;177(4):924–935. doi: 10.1111/bjd.15360 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials