Heterogeneity of Treatment Effects of Hydrocortisone by Risk of Bronchopulmonary Dysplasia or Death Among Extremely Preterm Infants in the National Institute of Child Health and Human Development Neonatal Research Network Trial: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 37256621
- PMCID: PMC10233424
- DOI: 10.1001/jamanetworkopen.2023.15315
Heterogeneity of Treatment Effects of Hydrocortisone by Risk of Bronchopulmonary Dysplasia or Death Among Extremely Preterm Infants in the National Institute of Child Health and Human Development Neonatal Research Network Trial: A Secondary Analysis of a Randomized Clinical Trial
Erratum in
-
Error in Table.JAMA Netw Open. 2023 Aug 1;6(8):e2331067. doi: 10.1001/jamanetworkopen.2023.31067. JAMA Netw Open. 2023. PMID: 37585208 Free PMC article. No abstract available.
Abstract
Importance: Extremely preterm infants who develop bronchopulmonary dysplasia (BPD) are at a higher risk for adverse pulmonary and neurodevelopmental outcomes. In the National Institute of Child Health and Human Development Neonatal Research Network (NICHD NRN) Hydrocortisone Trial, hydrocortisone neither reduced rates of BPD or death nor increased rates of neurodevelopmental impairment (NDI) or death.
Objective: To determine whether estimated risk for grades 2 to 3 BPD or death is associated with the effect of hydrocortisone on the composite outcomes of (1) grades 2 to 3 BPD or death and (2) moderate or severe NDI or death.
Design, setting, and participants: This secondary post hoc analysis used data from the NICHD NRN Hydrocortisone Trial, which was a double-masked, placebo-controlled, randomized clinical trial conducted in 19 US academic centers. The NICHD HRN Hydrocortisone Trial enrolled infants born at a gestational age of less than 30 weeks who received mechanical ventilation for at least 7 days, including at the time of enrollment, and who were aged 14 to 28 postnatal days. Infants were enrolled between August 22, 2011, and February 4, 2018, with follow-up between 22 and 26 months of corrected age completed on March 29, 2020. Data were analyzed from September 13, 2021, to March 25, 2023.
Intervention: Infants were randomized to 10 days of hydrocortisone or placebo treatment.
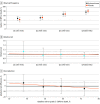
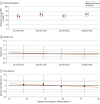
Main outcomes and measures: Infants' baseline risk of grades 2 to 3 BPD or death was estimated using the NICHD Neonatal BPD Outcome Estimator. Differences in absolute and relative treatment effects by baseline risk were evaluated using interaction terms in models fitted to the efficacy outcome of grades 2 to 3 BPD or death and the safety outcome of moderate or severe NDI or death by follow-up.
Results: Among the 799 infants included in the analysis (421 boys [52.7%]), the mean (SD) gestational age was 24.9 (1.5) weeks, and the mean (SD) birth weight was 715 (167) g. The mean estimated baseline risk for grades 2 to 3 BPD or death was 54% (range, 18%-84%) in the study population. The interaction between treatment group and baseline risk was not statistically significant on a relative or absolute scale for grades 2 to 3 BPD or death; the size of the effect ranged from a relative risk of 1.13 (95% CI, 0.82-1.55) in quartile 1 to 0.94 (95% CI, 0.81-1.09) in quartile 4. Similarly, the interaction between treatment group and baseline risk was not significant on a relative or absolute scale for moderate or severe NDI or death; the size of the effect ranged from a relative risk of 1.04 (95% CI, 0.80-1.36) in quartile 1 to 0.99 (95% CI, 0.80-1.22) in quartile 4.
Conclusions and relevance: In this secondary analysis of a randomized clinical trial, the effect of hydrocortisone vs placebo was not appreciably modified by baseline risk for grades 2 to 3 BPD or death.
Trial registration: ClinicalTrials.gov Identifier: NCT01353313.
Conflict of interest statement
Figures
Comment in
-
EBNEO Commentary: Does the effect of hydrocortisone in preterm infants depend on the baseline risk of BPD?Acta Paediatr. 2023 Dec;112(12):2620-2621. doi: 10.1111/apa.16977. Epub 2023 Sep 22. Acta Paediatr. 2023. PMID: 37740525 Free PMC article. No abstract available.
References
-
- Stoll BJ, Hansen NI, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039-1051. doi:10.1001/jama.2015.10244 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

