Population pharmacokinetics of benznidazole in neonates, infants and children using a new pediatric formulation
- PMID: 37256863
- PMCID: PMC10259795
- DOI: 10.1371/journal.pntd.0010850
Population pharmacokinetics of benznidazole in neonates, infants and children using a new pediatric formulation
Abstract
Background: There is a major need for information on pharmacokinetics (PK) of benznidazole (BNZ) in children with Chagas disease (CD). We conducted a multicentre population PK, safety and efficacy study in children, infants and neonates with CD treated with BNZ (formulated in 100 mg tablets or 12.5 mg dispersible tablets, developed by the pharmaceutical company LAFEPE, in a collaboration with DNDi).
Methods: 81 children 0-12 years old were enrolled at 5 pediatric centers in Argentina. Diagnosis of T. cruzi infection was confirmed by direct microscopic examination, or at least two positive conventional serological tests. Subject enrolment was stratified by age: newborns to 2 years (minimum of 10 newborns) and >2-12 years. BNZ 7.5 mg/kg/d was administered in two daily doses for 60 days. Five blood samples per child were obtained at random times within pre-defined time windows at Day 0 at 2-5 h post-dose; during steady state, one sample at Day 7 and at Day 30; and two samples at 12-24 h after final BNZ dose at Day 60. The primary efficacy endpoint was parasitological clearance by qualitative PCR at the end of treatment.
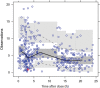
Results: Forty-one (51%) patients were under 2 years of age (including 14 newborns <1 month of age). Median age at enrolment was 22 months (mean: 43.2; interquartile range (IQR) 7-72 months). The median measured BNZ Cmax was 8.32 mg/L (IQR 5.95-11.8; range 1.79-19.38). Median observed BNZ Cmin (trough) concentration was 2 mg/L (IQR 1.25-3.77; range 0.14-7.08). Overall median simulated Css was 6.3 mg/L (IQR 4.7-8.5 mg/L). CL/F increased quickly during the first month of postnatal life and reached adult levels after approximately 10 years of age. Negative qPCR was observed at the end of treatment in all 76 patients who completed the treatment. Five patients discontinued treatment (3 due to AEs and 2 due to lack of compliance).
Conclusion: We observed lower BNZ plasma concentrations in infants and children than those previously reported in adults treated with comparable mg/kg doses. Despite these lower concentrations, pediatric treatment was well tolerated and universally effective, with a high response rate and infrequent, mild AEs.
Trial registration: Registered in clinicaltrials.gov #NCT01549236.
Copyright: © 2023 Altcheh et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Altcheh J, Moscatelli G, Mastrantonio G, Moroni S, Giglio N, Marson ME et al. Population pharmacokinetic study of benznidazole in pediatric Chagas disease suggests efficacy despite lower plasma concentrations than in adults. PLoS.Negl.Trop.Dis. 2014;8:e2907. doi: 10.1371/journal.pntd.0002907 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

