Airway proteolytic control of pneumococcal competence
- PMID: 37256908
- PMCID: PMC10259803
- DOI: 10.1371/journal.ppat.1011421
Airway proteolytic control of pneumococcal competence
Abstract
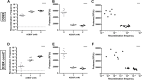
Streptococcus pneumoniae is an opportunistic pathogen that colonizes the upper respiratory tract asymptomatically and, upon invasion, can lead to severe diseases including otitis media, sinusitis, meningitis, bacteremia, and pneumonia. One of the first lines of defense against pneumococcal invasive disease is inflammation, including the recruitment of neutrophils to the site of infection. The invasive pneumococcus can be cleared through the action of serine proteases generated by neutrophils. It is less clear how serine proteases impact non-invasive pneumococcal colonization, which is the key first step to invasion and transmission. One significant aspect of pneumococcal biology and adaptation in the respiratory tract is its natural competence, which is triggered by a small peptide CSP. In this study, we investigate if serine proteases are capable of degrading CSP and the impact this has on pneumococcal competence. We found that CSP has several potential sites for trypsin-like serine protease degradation and that there were preferential cleavage sites recognized by the proteases. Digestion of CSP with two different trypsin-like serine proteases dramatically reduced competence in a dose-dependent manner. Incubation of CSP with mouse lung homogenate also reduced recombination frequency of the pneumococcus. These ex vivo experiments suggested that serine proteases in the lower respiratory tract reduce pneumococcal competence. This was subsequently confirmed measuring in vivo recombination frequencies after induction of protease production via poly (I:C) stimulation and via co-infection with influenza A virus, which dramatically lowered recombination events. These data shed light on a new mechanism by which the host can modulate pneumococcal behavior and genetic exchange via direct degradation of the competence signaling peptide.
Copyright: © 2023 Echlin et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures









References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

