Variant STAT4 and Response to Ruxolitinib in an Autoinflammatory Syndrome
- PMID: 37256972
- PMCID: PMC10392571
- DOI: 10.1056/NEJMoa2202318
Variant STAT4 and Response to Ruxolitinib in an Autoinflammatory Syndrome
Abstract
Background: Disabling pansclerotic morphea (DPM) is a rare systemic inflammatory disorder, characterized by poor wound healing, fibrosis, cytopenias, hypogammaglobulinemia, and squamous-cell carcinoma. The cause is unknown, and mortality is high.
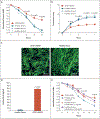
Methods: We evaluated four patients from three unrelated families with an autosomal dominant pattern of inheritance of DPM. Genomic sequencing independently identified three heterozygous variants in a specific region of the gene that encodes signal transducer and activator of transcription 4 (STAT4). Primary skin fibroblast and cell-line assays were used to define the functional nature of the genetic defect. We also assayed gene expression using single-cell RNA sequencing of peripheral-blood mononuclear cells to identify inflammatory pathways that may be affected in DPM and that may respond to therapy.
Results: Genome sequencing revealed three novel heterozygous missense gain-of-function variants in STAT4. In vitro, primary skin fibroblasts showed enhanced interleukin-6 secretion, with impaired wound healing, contraction of the collagen matrix, and matrix secretion. Inhibition of Janus kinase (JAK)-STAT signaling with ruxolitinib led to improvement in the hyperinflammatory fibroblast phenotype in vitro and resolution of inflammatory markers and clinical symptoms in treated patients, without adverse effects. Single-cell RNA sequencing revealed expression patterns consistent with an immunodysregulatory phenotype that were appropriately modified through JAK inhibition.
Conclusions: Gain-of-function variants in STAT4 caused DPM in the families that we studied. The JAK inhibitor ruxolitinib attenuated the dermatologic and inflammatory phenotype in vitro and in the affected family members. (Funded by the American Academy of Allergy, Asthma, and Immunology Foundation and others.).
Copyright © 2023 Massachusetts Medical Society.
Figures




Comment in
-
Variant STAT4 and Treatment of an Autoinflammatory Syndrome.N Engl J Med. 2023 Sep 21;389(12):1151. doi: 10.1056/NEJMc2308588. N Engl J Med. 2023. PMID: 37733320 No abstract available.
-
Variant STAT4 and Treatment of an Autoinflammatory Syndrome. Reply.N Engl J Med. 2023 Sep 21;389(12):1151-1152. doi: 10.1056/NEJMc2308588. N Engl J Med. 2023. PMID: 37733321 No abstract available.
References
-
- Wollina U, Looks A, Uhlemann C, Wollina K. Pansclerotic morphea of childhood-follow-up over 6 years. Pediatr Dermatol 1999;16:2 45–7. - PubMed
-
- Moll M, Holzer U, Zimmer C, Rieber N, Kuemmerle-Deschner JB. Autologous stem cell transplantation in two children with disabling pansclerotic morphea. Pediatr Rheumatol Online J 2011; 9: Suppl 1:77. abstract.
-
- Gruss C, Stücker M, Kobyletzki G, Schreiber D, Altmeyer P, Kerscher M. Low dose UVA1 phototherapy in disabling pansclerotic morphoea of childhood. Br J Dermatol 1997;136:293–4. - PubMed
-
- Kowal-Bielecka O, Distler O. Use of methotrexate in patients with scleroderma and mixed connective tissue disease. Clin Exp Rheumatol 2010;2 8: Suppl 61:S160–S163. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- National Human Genome Research Institute, Intramur/NH/NIH HHS/United States
- T32 GM008806/GM/NIGMS NIH HHS/United States
- UL1TR001442/NH/NIH HHS/United States
- UL1 TR001442/TR/NCATS NIH HHS/United States
- National Institute of Arthritis and Musculoskeleta/NH/NIH HHS/United States
- UH2 AI153029/AI/NIAID NIH HHS/United States
- S10 OD026929/OD/NIH HHS/United States
- UH2 AI153029/NH/NIH HHS/United States
- R35 GM119850/GM/NIGMS NIH HHS/United States
- FI2 GM137942/GM/NIGMS NIH HHS/United States
- Undiagnosed Diseases Network/NH/NIH HHS/United States
- T32GM8806/NH/NIH HHS/United States
- 1Fi2GM137942-01/NH/NIH HHS/United States
- R35 GM119850/NH/NIH HHS/United States
- ZID AR041180/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
