Understanding the Decline of Incident, Active Tuberculosis in People With Human Immunodeficiency Virus in Switzerland
- PMID: 37257071
- PMCID: PMC10640694
- DOI: 10.1093/cid/ciad330
Understanding the Decline of Incident, Active Tuberculosis in People With Human Immunodeficiency Virus in Switzerland
Abstract
Background: People with human immunodeficiency virus type 1 (HIV-1) (PWH) are frequently coinfected with Mycobacterium tuberculosis (MTB) and at risk for progressing from asymptomatic latent TB infection (LTBI) to active tuberculosis (TB). LTBI testing and preventive treatment (TB specific prevention) are recommended, but its efficacy in low transmission settings is unclear.
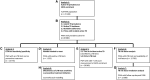
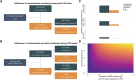
Methods: We included PWH enrolled from 1988 to 2022 in the Swiss HIV Cohort study (SHCS). The outcome, incident TB, was defined as TB ≥6 months after SHCS inclusion. We assessed its risk factors using a time-updated hazard regression, modeled the potential impact of modifiable factors on TB incidence, performed mediation analysis to assess underlying causes of time trends, and evaluated preventive measures.
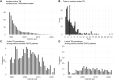
Results: In 21 528 PWH, LTBI prevalence declined from 15.1% in 2001% to 4.6% in 2021. Incident TB declined from 90.8 cases/1000 person-years in 1989 to 0.1 in 2021. A positive LTBI test showed a higher risk for incident TB (hazard ratio [HR] 9.8, 5.8-16.5) but only 10.5% of PWH with incident TB were tested positive. Preventive treatment reduced the risk in LTBI test positive PWH for active TB (relative risk reduction, 28.1%, absolute risk reduction 0.9%). On population level, the increase of CD4 T-cells and reduction of HIV viral load were the main driver of TB decrease.
Conclusions: TB specific prevention is effective in selected patient groups. On a population level, control of HIV-1 remains the most important factor for incident TB reduction. Accurate identification of PWH at highest risk for TB is an unmet clinical need.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest . A. C. received grants from Merck Sharp & Dohme (MSD), ViiV Healthcare, and Gilead Sciences for unrelated research. R. D. K. received grants from Gilead Sciences and National Institutes of Health (NIH) for unrelated research. D. L. B. received honoraria for working on the advisory board of Gilead Sciences, Merck, ViiV, Pfizer, and AstraZeneca. D. L. B. received honoraria for presentations from Gilead Sciences and Merck. E. B. received grants from MSD for unrelated research. E. B. received payments for travel reimbursement from ViiV, MSD, Gilead Sciences, Pfizer, and Abbvie. E. B. received honoraria for working on the advisory board of ViiV, MSD, Pfizer, Gilead Sciences, AstraZeneca, and Ely Lilly. H. H. H. received honoraria for working on the advisory board of AiCuris, Merck, Vera Dx, and Molecular Partners. H. H. H. received honoraria for presentations from Merck, Gilead Sciences, Biotest, and Vera Dx. J. N. received honoraria for presentations from Oxford Immunotec and ViiV. H. F. G. received honoraria for working on the advisory board of Gilead Sciences, Merck, ViiV, Janssen, Johnson and Johnson, Novartis, and GlaxoSmithKline (GSK). H. F. G. received payments for travel reimbursements from Gilead Sciences. H. F. G. received grants from NIH, Yvonne Jacob Foundation, and Gilead Sciences. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Goletti D, Weissman D, Jackson RW, et al. . Effect of Mycobacterium tuberculosis on HIV replication: role of immune activation. J Immunol 1996; 157:1271 LP–1278. - PubMed
-
- World Health Organization . 2021WHO Global TB Report. 2021.
-
- Wood R, Maartens G, Lombard CJ. Risk factors for developing tuberculosis in HIV-1-infected adults from communities with a low or very high incidence of tuberculosis. J Acquir Immune Defic Syndr 2000; 23:75–80. - PubMed
-
- Kumar P, Sharma N, Sharma NC, Patnaik S. Clinical profile of tuberculosis in patients with HIV infection/AIDS. Indian J Chest Dis Allied Sci 2002; 44:159–63. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

