Single CAR-T cell treatment controls disseminated ovarian cancer in a syngeneic mouse model
- PMID: 37258040
- PMCID: PMC10255004
- DOI: 10.1136/jitc-2022-006509
Single CAR-T cell treatment controls disseminated ovarian cancer in a syngeneic mouse model
Abstract
Background: Treatment of some blood cancers with T cells that express a chimeric antigen receptor (CAR) against CD19 have shown remarkable results. In contrast, CAR-T cell efficacy against solid tumors has been difficult to achieve.
Methods: To examine the potential of CAR-T cell treatments against ovarian cancers, we used the mouse ovarian cancer cell line ID8 in an intraperitoneal model that exhibits disseminated solid tumors in female C57BL/6J mice. The CAR contained a single-chain Fv from antibody 237 which recognizes a Tn-glycopeptide-antigen expressed by ID8 due to aberrant O-linked glycosylation in the absence of the transferase-dependent chaperone Cosmc. The efficacy of four Tn-dependent CARs with varying affinity to Tn antigen, and each containing CD28/CD3ζ cytoplasmic domains, were compared in vitro and in vivo in this study.
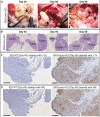
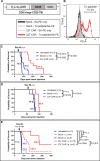
Results: In line with many observations about the impact of aberrant O-linked glycosylation, the ID8Cosmc knock-out (ID8Cosmc-KO) exhibited more rapid tumor progression compared with wild-type ID8. Despite the enhanced tumor growth in vivo, 237 CAR and a mutant with 30-fold higher affinity, but not CARs with lower affinity, controlled advanced ID8Cosmc-KO tumors. Tumor regression could be achieved with a single intravenous dose of the CARs, but intraperitoneal administration was even more effective. The CAR-T cells persisted over a period of months, allowing CAR-treated mice to delay tumor growth in a re-challenge setting. The most effective CARs exhibited the highest affinity for antigen. Antitumor effects observed in vivo were associated with increased numbers of T cells and macrophages, and higher levels of cleaved caspase-3, in the tumor microenvironment. Notably, the least therapeutically effective CAR mediated tonic signaling leading to antigen-independent cytokine expression and it had higher levels of the immunosuppressive cytokine interleukin10.
Conclusion: The findings support the development of affinity-optimized CAR-T cells as a potential treatment for established ovarian cancer, with the most effective CARs mediating a distinct pattern of inflammatory cytokine release in vitro. Importantly, the most potent Tn-dependent CAR-T cells showed no evidence of toxicity in tumor-bearing mice in a syngeneic, immunocompetent system.
Keywords: Antigens, Tumor-Associated, Carbohydrate; Cell Engineering; Immunotherapy; Receptors, Chimeric Antigen; T-Lymphocytes.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DMK is a member of the scientific advisory board of Affini-T Therapeutics and a consultant for Tempus. PS, HS, KS, and DMK are co-inventors on a patent application related to these chimeric antigen receptors. No potential conflicts of interest were disclosed by the other authors.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
