Landscape of cancer biomarker testing in England following genomic services reconfiguration: insights from a nationwide pathologist survey
- PMID: 37258251
- PMCID: PMC11228219
- DOI: 10.1136/jcp-2023-208890
Landscape of cancer biomarker testing in England following genomic services reconfiguration: insights from a nationwide pathologist survey
Abstract
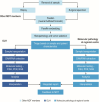
Aims: Cancer diagnostics have been evolving rapidly. In England, the new National Health Service Genomic Medicine Service (GMS) provides centralised access to genomic testing via seven regional Genomic Laboratory Hubs. The PATHways survey aimed to capture pathologists' experience with current diagnostic pathways and opportunities for optimisation to ensure equitable and timely access to biomarker testing.
Methods: A nationwide survey was conducted with consultant pathologists from regional laboratories, via direct interviews based on a structured questionnaire. Descriptive analysis of responses was undertaken using quantitative and qualitative methods.
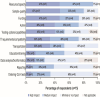
Results: Fifteen regional centres completed the survey covering a median population size of 2.5 (1.9-3.6) million (each for n=12). The median estimated turnaround time (calendar days) for standard molecular markers in melanoma, breast and lung cancers ranged from 2 to 3 days by immunohistochemistry (excluding NTRKfus in breast and lung cancers, and PD-L1 in melanoma) and 6-15 days by real-time-PCR (excluding KIT for melanoma), to 17.5-24.5 days by next-generation sequencing (excluding PIK3CA for breast cancer). Tests were mainly initiated by pathologists and oncologists. All respondents discussed the results at multidisciplinary team (MDT) meetings. The GMS roll-out was perceived to have high impact on services by 53% of respondents, citing logistical and technical issues. Enhanced education on new pathways, tissue requirements, report interpretation, providing patient information and best practice sharing was suggested for pathologists and other MDT members.
Conclusion: Our survey highlighted the role of regional pathology within the evolving diagnostic landscape in England. Notable recommendations included improved communication and education, active stakeholder engagement, and tackling informatics barriers.
Keywords: CANCER; DIAGNOSTICS; Health Services.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PT consultancy for AstraZeneca, Roche, Boehringer Ingelheim, and Qiagen. AN reportsreceiving personal fees from Merck, Boehringer Ingelheim, Novartis, AstraZeneca, Bristol-MyersSquibb, Roche, AbbVie, Oncologica, UptoDate, European Society of Oncology, Liberum, andTakeda UK and grants and personal fees from Pfizer, outside of the submitted work. JRG received grants from the Medical Research Council (MRC) and Eli Lilly and Company during theconduct of the study, and personal fees from AbbVie, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Diaceutics, Eli Lilly and Company, Merck Sharp & Dohme, Novartis, Pfizer, Roche and Takeda Oncology outside the submitted work. EV has provided experttestimony for Abbvie. DAM has received speaker fees from AstraZeneca and Takeda,consultancy fees from AstraZeneca, Thermo Fisher, Takeda, Amgen, Janssen, MIM Software,Bristol-Myers Squibb and Eli Lilly and educational support from Takeda and Amgen. LJ, IS, NB, DB, DAM, and PV have no conflicts of interest. AGL, RB and JR are employees of Novartis Ltd UK.
Figures


Similar articles
-
Molecular Pathology of Lung Cancer.Surg Pathol Clin. 2021 Sep;14(3):369-377. doi: 10.1016/j.path.2021.05.002. Epub 2021 Jul 8. Surg Pathol Clin. 2021. PMID: 34373089 Review.
-
Challenges in diagnosis and biomarker testing for RET-altered lung and thyroid cancer care: an international mixed-method study.BMC Med Educ. 2023 Jun 5;23(1):410. doi: 10.1186/s12909-023-04396-w. BMC Med Educ. 2023. PMID: 37277734 Free PMC article.
-
The implementation of molecular tumor profiling in the practice of pediatric cancer pathology: The pathologists' experience.Pediatr Blood Cancer. 2025 Jan;72(1):e31370. doi: 10.1002/pbc.31370. Epub 2024 Oct 10. Pediatr Blood Cancer. 2025. PMID: 39387390
-
Virtually the same? Examining the impact of the COVID-19 related shift to virtual lung cancer multidisciplinary team meetings in the UK National Health Service: a mixed methods study.BMJ Open. 2023 Jun 16;13(6):e065494. doi: 10.1136/bmjopen-2022-065494. BMJ Open. 2023. PMID: 37328174 Free PMC article.
-
The leading role of pathology in assessing the somatic molecular alterations of cancer: Position Paper of the European Society of Pathology.Virchows Arch. 2020 Apr;476(4):491-497. doi: 10.1007/s00428-020-02757-0. Epub 2020 Mar 2. Virchows Arch. 2020. PMID: 32124002 Free PMC article. Review.
Cited by
-
Genomic testing for RET in the clinic: UK and global perspective.Endocr Relat Cancer. 2025 Apr 15;32(5):e240230. doi: 10.1530/ERC-24-0230. Print 2025 May 1. Endocr Relat Cancer. 2025. PMID: 40138217 Free PMC article. Review.
-
Barriers and facilitators to next-generation sequencing use in United States oncology settings: a systematic review.Future Oncol. 2024;20(35):2765-2777. doi: 10.1080/14796694.2024.2390821. Epub 2024 Sep 24. Future Oncol. 2024. PMID: 39316553 Free PMC article.
-
Future perspective for the application of predictive biomarker testing in advanced stage non-small cell lung cancer.Lancet Reg Health Eur. 2024 Mar 1;38:100839. doi: 10.1016/j.lanepe.2024.100839. eCollection 2024 Mar. Lancet Reg Health Eur. 2024. PMID: 38476751 Free PMC article. Review.
References
-
- NHS Genomic medicine service. 2022. Available: https://www.england.nhs.uk/genomics
-
- The National Genomic test directory. 2022. Available: https://www.england.nhs.uk/genomics/the-national-genomic-test-directory
-
- Barwell J, Snape K, Wedderburn S. The new Genomic medicine service and implications for patients . Clin Med (Lond) 2019;19:273–7. 10.7861/clinmedicine.19-4-273 Available: mid: https://pubmed.ncbi.nlm.nih.gov/31308102/ - DOI - PMC - PubMed
-
- Pathology networks. 2022. Available: https://www.england.nhs.uk/pathology-networks
-
- Population estimates. 2022. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigrati...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
