Determinants of adherence to daily PrEP measured as intracellular tenofovir diphosphate concentrations over 24 months of follow-up among men who have sex with men
- PMID: 37258273
- PMCID: PMC10359585
- DOI: 10.1136/sextrans-2022-055499
Determinants of adherence to daily PrEP measured as intracellular tenofovir diphosphate concentrations over 24 months of follow-up among men who have sex with men
Abstract
Objectives: Adherence is key to the effectiveness of oral pre-exposure prophylaxis (PrEP) to prevent HIV. Therefore, we aimed to explore factors associated with adherence to daily PrEP (dPrEP).
Methods: Men who have sex with men (MSM) using dPrEP (emtricitabine/tenofovir disoproxil) within the Amsterdam PrEP demonstration project at the Public Health Service of Amsterdam, provided dried blood spots (DBS) 12 and 24 months after PrEP initiation. From DBS, we determined intracellular tenofovir diphosphate (TFV-DP) concentrations to assess adherence; TFV-DP ≥700 fmol/punch was considered adequate. We assessed associations of sociodemographic, clinical and behavioural characteristics with TFV-DP concentrations using multivariable linear regression.
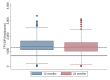
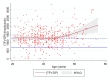
Results: Of 263 participants who attended 12-month or 24-month study visits while on dPrEP, 257 (97.7%) provided DBS at one or both visits (492 DBS in total). Median TFV-DP concentration was 1299 (IQR 1021-1627) fmol/punch (12 months: 1332 (1087-1687); 24 months: 1248 (929-1590]). Higher TFV-DP concentrations were associated with: older age (p=0.0008), condomless anal sex with a casual partner in 6 months preceding PrEP initiation (+166 fmol/punch; 95% CI 36.5 to 296) and using a mobile application providing visualised feedback on PrEP use and sexual behaviour (+146 fmol/punch; 95% CI 28.1 to 263). Lower TFV-DP concentrations were associated with longer duration of PrEP use (24 vs 12 months; -91.5 fmol/punch; 95% CI -155 to -28.1). Time-updated number of sex partners, diagnosed STIs and chemsex were not associated with TFV-DP concentrations.
Conclusions: Overall, TFV-DP concentrations were high among MSM using dPrEP, indicating excellent adherence. Especially older participants, those who reported condomless anal sex with a casual partner prior to PrEP initiation and those who used an app with visualised feedback showed higher levels of adherence. As TFV-DP concentrations had decreased slightly at 2 years of PrEP use when compared with 1 year, we emphasise the importance of adherence counselling to those who continue using PrEP.
Trial registration number: NL5413.
Keywords: HIV; cohort studies; pre-exposure prophylaxis; treatment adherence and compliance.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: The study medication was provided by Gilead Sciences based on unconditional grants. EH received advisory board fees from Gilead Sciences and speaker fees from Janssen‐Cilag, both paid to her institute. UD received unrestricted research grants and speaker’s fees from Gilead Sciences, paid to his institute. HJCdV received grants from Medigene, and advisory board and speaker fees from Gilead Sciences, Medigene, AbbVie, Janssen‐Cilag and Willpharma, paid to his institute. MP received unrestricted research grants and speaker’s fees from Gilead Sciences, Roche, AbbVie and MSD, paid to her institute. MSvdL served on an Advisory Board of MSD, paid to his institute. All other authors declared no competing interest.
Figures
References
-
- van Sighem AI, FWNM W, Boyd A, Smit C, Matser A, van der Valk M. Monitoring report 2021. Human immunodeficiency virus (HIV) infection in the Netherlands. Amsterdam: Stichting hiv monitoring; 2021.
-
- Dashwood T, Tan DHS. Preparing for the unexpected: mechanisms and management of HIV pre-exposure prophylaxis failure. Future Virol 2018;13:747–59. 10.2217/fvl-2018-0084 - DOI
-
- WHO . WHO implementation tool for pre-exposure prophylaxis of HIV infection. Geneva, Switzerland: World Health Organization; 2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
