Host-diet-gut microbiome interactions influence human energy balance: a randomized clinical trial
- PMID: 37258525
- PMCID: PMC10232526
- DOI: 10.1038/s41467-023-38778-x
Host-diet-gut microbiome interactions influence human energy balance: a randomized clinical trial
Abstract
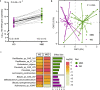
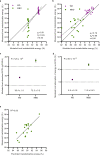
The gut microbiome is emerging as a key modulator of human energy balance. Prior studies in humans lacked the environmental and dietary controls and precision required to quantitatively evaluate the contributions of the gut microbiome. Using a Microbiome Enhancer Diet (MBD) designed to deliver more dietary substrates to the colon and therefore modulate the gut microbiome, we quantified microbial and host contributions to human energy balance in a controlled feeding study with a randomized crossover design in young, healthy, weight stable males and females (NCT02939703). In a metabolic ward where the environment was strictly controlled, we measured energy intake, energy expenditure, and energy output (fecal and urinary). The primary endpoint was the within-participant difference in host metabolizable energy between experimental conditions [Control, Western Diet (WD) vs. MBD]. The secondary endpoints were enteroendocrine hormones, hunger/satiety, and food intake. Here we show that, compared to the WD, the MBD leads to an additional 116 ± 56 kcals (P < 0.0001) lost in feces daily and thus, lower metabolizable energy for the host (89.5 ± 0.73%; range 84.2-96.1% on the MBD vs. 95.4 ± 0.21%; range 94.1-97.0% on the WD; P < 0.0001) without changes in energy expenditure, hunger/satiety or food intake (P > 0.05). Microbial 16S rRNA gene copy number (a surrogate of biomass) increases (P < 0.0001), beta-diversity changes (whole genome shotgun sequencing; P = 0.02), and fermentation products increase (P < 0.01) on an MBD as compared to a WD along with significant changes in the host enteroendocrine system (P < 0.0001). The substantial interindividual variability in metabolizable energy on the MBD is explained in part by fecal SCFAs and biomass. Our results reveal the complex host-diet-microbiome interplay that modulates energy balance.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Update of
-
Reprogramming the Human Gut Microbiome Reduces Dietary Energy Harvest.Res Sq [Preprint]. 2023 Jan 25:rs.3.rs-2382790. doi: 10.21203/rs.3.rs-2382790/v1. Res Sq. 2023. Update in: Nat Commun. 2023 May 31;14(1):3161. doi: 10.1038/s41467-023-38778-x. PMID: 36747835 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

