Design and evaluation of a skin-on-a-chip pumpless microfluidic device
- PMID: 37258538
- PMCID: PMC10232512
- DOI: 10.1038/s41598-023-34796-3
Design and evaluation of a skin-on-a-chip pumpless microfluidic device
Abstract
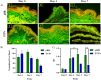
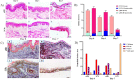
The development of microfluidic culture technology facilitates the progress of study of cell and tissue biology. This technology expands the understanding of pathological and physiological changes. A skin chip, as in vitro model, consisting of normal skin tissue with epidermis and dermis layer (full thickness) was developed. Polydimethylsiloxane microchannels with a fed-batched controlled perfusion feeding system were used to create a full-thick ex-vivo human skin on-chip model. The design of a novel skin-on-a-chip model was reported, in which the microchannel structures mimic the architecture of the realistic vascular network as nutrients transporter to the skin layers. Viabilities of full-thick skin samples cultured on the microbioreactor and traditional tissue culture plate revealed that a precise controlled condition provided by the microfluidic enhanced tissue viability at least for seven days. Several advantages in skin sample features under micro-scale-controlled conditions were found such as skin mechanical strength, water adsorption, skin morphology, gene expression, and biopsy longevity. This model can provide an in vitro environment for localizing drug delivery and transdermal drug diffusion studies. The skin on the chip can be a valuable in vitro model for representing the interaction between drugs and skin tissue and a realistic platform for evaluating skin reaction to pharmaceutical materials and cosmetic products.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Testing the Effectiveness of Curcuma longa Leaf Extract on a Skin Equivalent Using a Pumpless Skin-on-a-Chip Model.Int J Mol Sci. 2020 May 29;21(11):3898. doi: 10.3390/ijms21113898. Int J Mol Sci. 2020. PMID: 32486109 Free PMC article.
-
Construction of 3D multicellular microfluidic chip for an in vitro skin model.Biomed Microdevices. 2017 Jun;19(2):22. doi: 10.1007/s10544-017-0156-5. Biomed Microdevices. 2017. PMID: 28374277
-
Effect of α-Lipoic Acid on the Development of Human Skin Equivalents Using a Pumpless Skin-on-a-Chip Model.Int J Mol Sci. 2021 Feb 22;22(4):2160. doi: 10.3390/ijms22042160. Int J Mol Sci. 2021. PMID: 33671528 Free PMC article.
-
Microfluidic Organ-on-a-Chip System for Disease Modeling and Drug Development.Biosensors (Basel). 2022 May 27;12(6):370. doi: 10.3390/bios12060370. Biosensors (Basel). 2022. PMID: 35735518 Free PMC article. Review.
-
Next generation human skin constructs as advanced tools for drug development.Exp Biol Med (Maywood). 2017 Nov;242(17):1657-1668. doi: 10.1177/1535370217712690. Epub 2017 Jun 7. Exp Biol Med (Maywood). 2017. PMID: 28592171 Free PMC article. Review.
Cited by
-
Emergent trends in organ-on-a-chip applications for investigating metastasis within tumor microenvironment: A comprehensive bibliometric analysis.Heliyon. 2023 Dec 9;10(1):e23504. doi: 10.1016/j.heliyon.2023.e23504. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38187238 Free PMC article.
-
Environmentally Controlled Microfluidic System Enabling Immune Cell Flow and Activation in an Endothelialised Skin-On-Chip.Adv Healthc Mater. 2024 Nov;13(29):e2400750. doi: 10.1002/adhm.202400750. Epub 2024 Oct 6. Adv Healthc Mater. 2024. PMID: 39370595 Free PMC article.
-
Advancements of paper-based microfluidics and organ-on-a-chip models in cosmetics hazards.RSC Adv. 2025 Apr 3;15(13):10319-10335. doi: 10.1039/d4ra07336c. eCollection 2025 Mar 28. RSC Adv. 2025. PMID: 40182506 Free PMC article. Review.
-
Transdermal Drug Delivery Systems: Methods for Enhancing Skin Permeability and Their Evaluation.Pharmaceutics. 2025 Jul 20;17(7):936. doi: 10.3390/pharmaceutics17070936. Pharmaceutics. 2025. PMID: 40733144 Free PMC article. Review.
-
Report of the Assay Guidance Workshop on 3-Dimensional Tissue Models for Antiviral Drug Development.J Infect Dis. 2023 Oct 3;228(Suppl 5):S337-S354. doi: 10.1093/infdis/jiad334. J Infect Dis. 2023. PMID: 37669225 Free PMC article.
References
-
- Kanitakis J. Anatomy, histology, and immunohistochemistry of normal human skin. Eur. J. Dermatol. 2002;12:390–401. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

