Allantoic fluid metabolome reveals specific metabolic signatures in chicken lines different for their muscle glycogen content
- PMID: 37258592
- PMCID: PMC10232533
- DOI: 10.1038/s41598-023-35652-0
Allantoic fluid metabolome reveals specific metabolic signatures in chicken lines different for their muscle glycogen content
Abstract
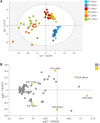
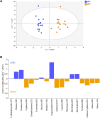
Nutrient availability in eggs can affect early metabolic orientation in birds. In chickens divergently selected on the Pectoralis major ultimate pH, a proxy for muscle glycogen stores, characterization of the yolk and amniotic fluid revealed a different nutritional environment. The present study aimed to assess indicators of embryo metabolism in pHu lines (pHu+ and pHu-) using allantoic fluids (compartment storing nitrogenous waste products and metabolites), collected at days 10, 14 and 17 of embryogenesis and characterized by 1H-NMR spectroscopy. Analysis of metabolic profiles revealed a significant stage effect, with an enrichment in metabolites at the end of incubation, and an increase in interindividual variability during development. OPLS-DA analysis discriminated the two lines. The allantoic fluid of pHu- was richer in carbohydrates, intermediates of purine metabolism and derivatives of tryptophan-histidine metabolism, while formate, branched-chain amino acids, Krebs cycle intermediates and metabolites from different catabolic pathways were more abundant in pHu+. In conclusion, the characterization of the main nutrient sources for embryos and now allantoic fluids provided an overview of the in ovo nutritional environment of pHu lines. Moreover, this study revealed the establishment, as early as day 10 of embryo development, of specific metabolic signatures in the allantoic fluid of pHu+ and pHu- lines.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

