Effects of three microtubule-associated proteins (MAP2, MAP4, and Tau) on microtubules' physical properties and neurite morphology
- PMID: 37258650
- PMCID: PMC10232483
- DOI: 10.1038/s41598-023-36073-9
Effects of three microtubule-associated proteins (MAP2, MAP4, and Tau) on microtubules' physical properties and neurite morphology
Abstract
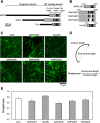
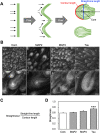
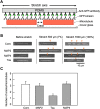
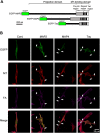
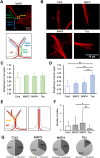
The physical properties of cytoskeletal microtubules have a multifaceted effect on the expression of their cellular functions. A superfamily of microtubule-associated proteins, MAP2, MAP4, and tau, promote the polymerization of microtubules, stabilize the formed microtubules, and affect the physical properties of microtubules. Here, we show differences in the effects of these three MAPs on the physical properties of microtubules. When microtubule-binding domain fragments of MAP2, tau, and three MAP4 isoforms were added to microtubules in vitro and observed by fluorescence microscopy, tau-bound microtubules showed a straighter morphology than the microtubules bound by MAP2 and the three MAP4 isoforms. Flexural rigidity was evaluated by the shape of the teardrop pattern formed when microtubules were placed in a hydrodynamic flow, revealing that tau-bound microtubules were the least flexible. When full-length MAPs fused with EGFP were expressed in human neuroblastoma (SH-SY5Y) cells, the microtubules in apical regions of protrusions expressing tau were straighter than in cells expressing MAP2 and MAP4. On the other hand, the protrusions of tau-expressing cells had the fewest branches. These results suggest that the properties of microtubules, which are regulated by MAPs, contribute to the morphogenesis of neurites.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Alberts B, et al. Molecular Biology of the Cell. 6. Garland Science; 2014.
-
- McNally FJ, Vale RD. Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell. 1993;75:419–429. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

