Astrocytic pathology in Alpers' syndrome
- PMID: 37259148
- PMCID: PMC10230702
- DOI: 10.1186/s40478-023-01579-w
Astrocytic pathology in Alpers' syndrome
Abstract
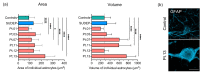
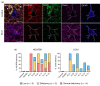
Refractory epilepsy is the main neurological manifestation of Alpers' syndrome, a severe childhood-onset mitochondrial disease caused by bi-allelic pathogenic variants in the mitochondrial DNA (mtDNA) polymerase gamma gene (POLG). The pathophysiological mechanisms underpinning neuronal hyperexcitabilty leading to seizures in Alpers' syndrome remain unknown. However, pathological changes to reactive astrocytes are hypothesised to exacerbate neural dysfunction and seizure-associated cortical activity in POLG-related disease. Therefore, we sought to phenotypically characterise astrocytic pathology in Alpers' syndrome. We performed a detailed quantitative investigation of reactive astrocytes in post-mortem neocortical tissues from thirteen patients with Alpers' syndrome, eight neurologically normal controls and five sudden unexpected death in epilepsy (SUDEP) patients, to control for generalised epilepsy-associated astrocytic pathology. Immunohistochemistry to identify glial fibrillary acidic protein (GFAP)-reactive astrocytes revealed striking reactive astrogliosis localised to the primary visual cortex of Alpers' syndrome tissues, characterised by abnormal-appearing hypertrophic astrocytes. Phenotypic characterisation of individual GFAP-reactive astrocytes demonstrated decreased abundance of mitochondrial oxidative phosphorylation (OXPHOS) proteins and altered expression of key astrocytic proteins including Kir4.1 (subunit of the inwardly rectifying K+ ion channel), AQP4 (astrocytic water channel) and glutamine synthetase (enzyme that metabolises glutamate). These phenotypic astrocytic changes were typically different from the pathology observed in SUDEP tissues, suggesting alternative mechanisms of astrocytic dysfunction between these epilepsies. Crucially, our findings provide further evidence of occipital lobe involvement in Alpers' syndrome and support the involvement of reactive astrocytes in the pathogenesis of POLG-related disease.
Keywords: Alpers’ syndrome; Aquaporin 4 (AQP4); GFAP; Glutamine synthetase (GS); Kir4.1; Mitochondrial Epilepsy; POLG; Reactive astrogliosis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Ropp PA, Copeland WC. Cloning and characterization of the human mitochondrial DNA polymerase, DNA polymerase gamma. Genomics. 1996;36(3):449–458. - PubMed
-
- Naviaux RK, Nguyen KV. POLG mutations associated with Alpers syndrome and mitochondrial DNA depletion. Ann Neurol. 2004;55(5):706–712. - PubMed
-
- Lim A, Thomas RH. The mitochondrial epilepsies. Eur J Paediatr Neurol. 2020;24:47–52. - PubMed
-
- Copeland WC, Human DNA Polymerase Gamma Mutation Database [Available from: https://tools.niehs.nih.gov/polg/index.cfm/main/home/limitresults/false
-
- Naviaux RK, Nyhan WL, Barshop BA, Poulton J, Markusic D, Karpinski NC, Haas RH. Mitochondrial DNA polymerase Gamma Deficiency and mtDNA Depletion in a child with Alpers’ syndrome. Annu Neurol. 1999;45:54–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

