Metabolism-Guided Optimization of Tryptophanol-Derived Isoindolinone p53 Activators
- PMID: 37259297
- PMCID: PMC9967700
- DOI: 10.3390/ph16020146
Metabolism-Guided Optimization of Tryptophanol-Derived Isoindolinone p53 Activators
Abstract
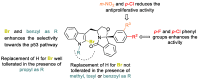
For the first time, the pharmacokinetic (PK) profile of tryptophanol-derived isoindolinones, previously reported as p53 activators, was investigated. From the metabolites' identification, performed by liquid chromatography coupled to high resolution tandem mass spectrometry (LC-HRMS/MS), followed by their preparation and structural elucidation, it was possible to identify that the indole C2 and C3 are the main target of the cytochrome P450 (CYP)-promoted oxidative metabolism in the tryptophanol-derived isoindolinone scaffold. Based on these findings, to search for novel p53 activators a series of 16 enantiopure tryptophanol-derived isoindolinones substituted with a bromine in indole C2 was prepared, in yields of 62-89%, and their antiproliferative activity evaluated in human colon adenocarcinoma HCT116 cell lines with and without p53. Structural optimization led to the identification of two (S)-tryptophanol-derived isoindolinones 3.9-fold and 1.9-fold more active than hit SLMP53-1, respectively. Compounds' metabolic stability evaluation revealed that this substitution led to a metabolic switch, with the impact of Phase I oxidative metabolism being minimized. Through differential scanning fluorimetry (DSF) experiments, the most active compound of the series in cell assays led to an increase in the protein melting temperature (Tm) of 10.39 °C, suggesting an effective binding to wild-type p53 core domain.
Keywords: cancer; hit-to-lead optimization; metabolic stability; p53; tryptophanol-derived isoindolinones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Van Cutsem E., Cervantes A., Adam R., Sobrero A., Van Krieken J.H., Aderka D., Aranda Aguilar E., Bardelli A., Benson A., Bodoky G., et al. ESMO Consensus Guidelines for the Management of Patients with Metastatic Colorectal Cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. - DOI - PubMed
-
- Liang Y.-H., Liang J.-T., Lin B.-R., Huang J., Hung J.-S., Lai S.-L., Chen T.-C., Tsai J.-H., Cheng Y.-M., Tsao T.-H., et al. Ramucirumab plus Triplet Chemotherapy as an Alternative Salvage Treatment for Patients with Metastatic Colorectal Cancer. J. Formos. Med. Assoc. 2022;121:2057–2064. doi: 10.1016/j.jfma.2022.02.019. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

