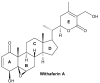
Withaferin A: A Pleiotropic Anticancer Agent from the Indian Medicinal Plant Withania somnifera (L.) Dunal
- PMID: 37259311
- PMCID: PMC9966696
- DOI: 10.3390/ph16020160
Withaferin A: A Pleiotropic Anticancer Agent from the Indian Medicinal Plant Withania somnifera (L.) Dunal
Abstract
Cancer represents the second most deadly disease and one of the most important public health concerns worldwide. Surgery, chemotherapy, radiation therapy, and immune therapy are the major types of treatment strategies that have been implemented in cancer treatment. Unfortunately, these treatment options suffer from major limitations, such as drug-resistance and adverse effects, which may eventually result in disease recurrence. Many phytochemicals have been investigated for their antitumor efficacy in preclinical models and clinical studies to discover newer therapeutic agents with fewer adverse effects. Withaferin A, a natural bioactive molecule isolated from the Indian medicinal plant Withania somnifera (L.) Dunal, has been reported to impart anticancer activities against various cancer cell lines and preclinical cancer models by modulating the expression and activity of different oncogenic proteins. In this article, we have comprehensively discussed the biosynthesis of withaferin A as well as its antineoplastic activities and mode-of-action in in vitro and in vivo settings. We have also reviewed the effect of withaferin A on the expression of miRNAs, its combinational effect with other cytotoxic agents, withaferin A-based formulations, safety and toxicity profiles, and its clinical potential.
Keywords: angiogenesis; apoptosis; cancer; chemoresistance; formulations; withaferin A.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Withania somnifera (L.) Dunal: Phytochemistry, structure-activity relationship, and anticancer potential.Phytomedicine. 2022 Apr;98:153949. doi: 10.1016/j.phymed.2022.153949. Epub 2022 Jan 19. Phytomedicine. 2022. PMID: 35151215 Review.
-
Review on anticancerous therapeutic potential of Withania somnifera (L.) Dunal.J Ethnopharmacol. 2021 Apr 24;270:113704. doi: 10.1016/j.jep.2020.113704. Epub 2020 Dec 25. J Ethnopharmacol. 2021. PMID: 33359918 Review.
-
Anticancer activities of Withania somnifera: Current research, formulations, and future perspectives.Pharm Biol. 2016;54(2):189-97. doi: 10.3109/13880209.2015.1027778. Epub 2015 Apr 7. Pharm Biol. 2016. PMID: 25845640 Review.
-
Distribution of withaferin A, an anticancer potential agent, in different parts of two varieties of Withania somnifera (L.) Dunal. grown in Sri Lanka.Pak J Biol Sci. 2013 Feb 1;16(3):141-4. doi: 10.3923/pjbs.2013.141.144. Pak J Biol Sci. 2013. PMID: 24171276
-
Withania somnifera - a magic plant targeting multiple pathways in cancer related inflammation.Phytomedicine. 2022 Jul;101:154137. doi: 10.1016/j.phymed.2022.154137. Epub 2022 May 2. Phytomedicine. 2022. PMID: 35533610 Review.
Cited by
-
Withaferin A Rescues Brain Network Dysfunction and Cognitive Deficits in a Mouse Model of Alzheimer's Disease.Pharmaceuticals (Basel). 2025 May 29;18(6):816. doi: 10.3390/ph18060816. Pharmaceuticals (Basel). 2025. PMID: 40573212 Free PMC article.
-
Synergistic Inhibition of Pancreatic Cancer Cell Growth and Migration by Gemcitabine and Withaferin A.Biomolecules. 2024 Sep 19;14(9):1178. doi: 10.3390/biom14091178. Biomolecules. 2024. PMID: 39334944 Free PMC article.
-
Withaferin A and Celastrol Overwhelm Proteostasis.Int J Mol Sci. 2023 Dec 27;25(1):367. doi: 10.3390/ijms25010367. Int J Mol Sci. 2023. PMID: 38203539 Free PMC article. Review.
-
Exploring the Potent Anticancer Activity of Essential Oils and Their Bioactive Compounds: Mechanisms and Prospects for Future Cancer Therapy.Pharmaceuticals (Basel). 2023 Jul 31;16(8):1086. doi: 10.3390/ph16081086. Pharmaceuticals (Basel). 2023. PMID: 37631000 Free PMC article. Review.
-
Oxidative Stress and Mitochondrial Dysfunction in Myelodysplastic Syndrome: Roles in Development, Diagnosis, Prognosis, and Treatment.Int J Mol Sci. 2025 Jul 3;26(13):6415. doi: 10.3390/ijms26136415. Int J Mol Sci. 2025. PMID: 40650192 Free PMC article. Review.
References
-
- Abadi A.J., Mirzaei S., Mahabady M.K., Hashemi F., Zabolian A., Hashemi F., Raee P., Aghamiri S., Ashrafizadeh M., Aref A.R., et al. Curcumin and its derivatives in cancer therapy: Potentiating antitumor activity of cisplatin and reducing side effects. Phytother. Res. 2022;36:189–213. doi: 10.1002/ptr.7305. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

