Topical Administration of a Nanoformulation of Chitosan-Hyaluronic Acid-Epoetin Beta in a Rat Model of Glaucoma
- PMID: 37259314
- PMCID: PMC9963646
- DOI: 10.3390/ph16020164
Topical Administration of a Nanoformulation of Chitosan-Hyaluronic Acid-Epoetin Beta in a Rat Model of Glaucoma
Abstract
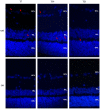
The present work investigates the effects of chitosan-hyaluronic acid-epoetin beta (CS/HA-EPOβ) nanoparticles after topical ocular administration in a rat glaucoma model. Wistar Hannover rats (n = 24) were submitted to a complete ophthalmological examination and electroretinography, followed by glaucoma induction in their right eye on day 1 of the study. Treatment group (T) received CS/HA-EPOβ nanocarriers (n = 12), while the control group (C) received only empty ones. Electroretinography was repeated on day 3 (n = 24) and before euthanasia on day 7 (n = 8), 14 (n = 8), and 21 (n = 8), followed by bilateral enucleation and histological assessment. The animals showed good tolerance to the nanoformulation. Maximum IOP values on the right eye occurred shortly after glaucoma induction (T = 62.6 ± 8.3 mmHg; C = 63.6 ± 7.9 mmHg). Animals from the treated group presented a tendency for faster recovery of retinal electrical activity (p > 0.05). EPOβ was detected on the retina of all treated eyes using immunofluorescence. Control animals presented with thinner retinas compared to the treated ones (p < 0.05). Therefore, topical ocular administration of CS/HA-EPOβ nanoparticles enabled EPOβ delivery to the retina of glaucomatous rats and promoted an earlier retinal recovery, confirming EPOβ's neuroprotective effects. The encouraging results of this preclinical study pave the way for new strategies for topical ocular administration of neuroprotective compounds.
Keywords: chitosan; epoetin beta; glaucoma; hyaluronic acid; nanoparticles; neuroprotection; ocular delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Vidal-Sanz M., Salinas-Navarro M., Nadal-Nicolás F.M., Alarcón-Martínez L., Valiente-Soriano F.J., Miralles de Imperial J., Avilés-Trigueros M., Agudo-Barriuso M., Villegas-Pérez M.P. Understanding glaucomatous damage: Anatomical and functional data from ocular hypertensive rodent retinas. Prog. Retin. Eye Res. 2012;31:1–27. doi: 10.1016/j.preteyeres.2011.08.001. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

