Treatment of Fabry Disease: Established and Emerging Therapies
- PMID: 37259462
- PMCID: PMC9967779
- DOI: 10.3390/ph16020320
Treatment of Fabry Disease: Established and Emerging Therapies
Abstract
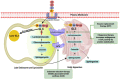
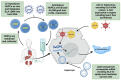
Fabry disease (FD) is a rare, X-linked inherited disorder of glycosphingolipid metabolism. It leads to the progressive accumulation of globotriaosylceramide within lysosomes due to a deficiency of α-galactosidase A enzyme. It involves multiple organs, predominantly the renal, cardiac, and cerebrovascular systems. Early diagnosis and treatment are critical to prevent progression to irreversible tissue damage and organ failure, and to halt life-threatening complications that can significantly reduce life expectancy. This review will focus on the established and emerging treatment options for FD.
Keywords: Fabry disease; chaperone therapy; enzyme replacement therapy; gene therapy; left ventricular hypertrophy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cardiac MRI in Fabry disease.Front Cardiovasc Med. 2023 Feb 2;9:1075639. doi: 10.3389/fcvm.2022.1075639. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36818911 Free PMC article. Review.
-
Anderson-Fabry disease: a multiorgan disease.Curr Pharm Des. 2013;19(33):5974-96. doi: 10.2174/13816128113199990352. Curr Pharm Des. 2013. PMID: 23448451 Review.
-
Cardiac Involvement in Fabry Disease and the Role of Multimodality Imaging in Diagnosis and Disease Monitoring.Curr Probl Cardiol. 2023 Jan;48(1):101439. doi: 10.1016/j.cpcardiol.2022.101439. Epub 2022 Oct 4. Curr Probl Cardiol. 2023. PMID: 36202174 Review.
-
Fabry disease: Definition, Incidence, Clinical presentations and Treatment - Focus on cardiac involvement.Pak J Med Sci. 2022 Nov-Dec;38(8):2337-2344. doi: 10.12669/pjms.38.8.7063. Pak J Med Sci. 2022. PMID: 36415271 Free PMC article. Review.
-
Paediatric Fabry disease.Transl Pediatr. 2016 Jan;5(1):37-42. doi: 10.3978/j.issn.2224-4336.2015.12.02. Transl Pediatr. 2016. PMID: 26835405 Free PMC article. Review.
Cited by
-
Exploring the burdens of women living with Fabry disease in Japan: A patient survey of 62 respondents.Mol Genet Metab Rep. 2025 May 30;43:101231. doi: 10.1016/j.ymgmr.2025.101231. eCollection 2025 Jun. Mol Genet Metab Rep. 2025. PMID: 40520913 Free PMC article.
-
In vivo applications and toxicities of AAV-based gene therapies in rare diseases.Orphanet J Rare Dis. 2025 Jul 17;20(1):368. doi: 10.1186/s13023-025-03893-z. Orphanet J Rare Dis. 2025. PMID: 40676625 Free PMC article. Review.
-
Human in vitro models for Fabry disease: new paths for unravelling disease mechanisms and therapies.J Transl Med. 2024 Oct 24;22(1):965. doi: 10.1186/s12967-024-05756-w. J Transl Med. 2024. PMID: 39449071 Free PMC article. Review.
-
Multidisciplinary Care Model as a Center of Excellence for Fabry Disease: A Practical Guide to Diagnosis and Management by Clinical Specialty in South Korea.J Clin Med. 2025 Jun 20;14(13):4400. doi: 10.3390/jcm14134400. J Clin Med. 2025. PMID: 40648776 Free PMC article. Review.
-
Green Biologics: Harnessing the Power of Plants to Produce Pharmaceuticals.Int J Mol Sci. 2023 Dec 17;24(24):17575. doi: 10.3390/ijms242417575. Int J Mol Sci. 2023. PMID: 38139405 Free PMC article. Review.
References
-
- Gersh B.J., Maron B.J., Bonow R.O., Dearani J.A., Fifer M.A., Link M.S., Naidu S.S., Nishimura R.A., Ommen S.R., Rakowski H., et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2011;58:2703–2738. doi: 10.1016/j.jacc.2011.10.825. - DOI - PubMed
-
- Mehta A., Hughes D.A. Fabry Disease. In: Adam M.P., Everman D.B., Mirzaa G.M., Pagon R.A., Wallace S.E., Bean L.J., Gripp K.W., Amemiya A., editors. GeneReviews. University of Washington; Seattle, WA, USA: 1993. [(accessed on 23 August 2022)]. Available online: http://www.ncbi.nlm.nih.gov/books/NBK1292/ - PubMed
-
- Eng C.M., Fletcher J., Wilcox W.R., Waldek S., Scott C.R., Sillence D.O., Breunig F., Charrow J., Germain D.P., Nicholls K., et al. Fabry disease: Baseline medical characteristics of a cohort of 1765 males and females in the Fabry Registry. J. Inherit. Metab. Dis. 2007;30:184–192. doi: 10.1007/s10545-007-0521-2. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

