CD47 Activation by Thrombospondin-1 in Lymphatic Endothelial Cells Suppresses Lymphangiogenesis and Promotes Atherosclerosis
- PMID: 37259865
- PMCID: PMC10281185
- DOI: 10.1161/ATVBAHA.122.318904
CD47 Activation by Thrombospondin-1 in Lymphatic Endothelial Cells Suppresses Lymphangiogenesis and Promotes Atherosclerosis
Abstract
Background: TSP1 (thrombospondin-1)-a well-known angiogenesis inhibitor-mediates differential effects via interacting with cell surface receptors including CD36 (cluster of differentiation) and CD47. However, the role of TSP1 in regulating lymphangiogenesis is not clear. Our previous study suggested the importance of cell-specific CD47 blockade in limiting atherosclerosis. Further, our experiments revealed CD47 as a dominant TSP1 receptor in lymphatic endothelial cells (LECs). As the lymphatic vasculature is functionally linked to atherosclerosis, we aimed to investigate the effects of LEC TSP1-CD47 signaling inhibition on lymphangiogenesis and atherosclerosis.
Methods: Murine atherosclerotic and nonatherosclerotic arteries were utilized to investigate TSP1 expression using Western blotting and immunostaining. LEC-specific knockout mice were used to determine the in vivo role of LEC Cd47 in lymphangiogenesis and atherosclerosis. Various in vitro cell-based assays, in vivo Matrigel plug implantation, molecular biological techniques, and immunohistological approaches were used to evaluate the underlying signaling mechanisms.
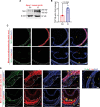
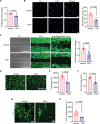
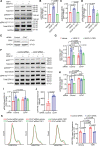
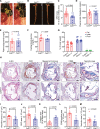
Results: Elevated TSP1 expression was observed in mouse atherosclerotic aortic tissue compared with nonatherosclerotic control tissue. TSP1 at pathological concentrations suppressed both in vitro and in vivo lymphangiogenesis. Mechanistically, TSP1 inhibited VEGF (vascular endothelial growth factor)-C-induced AKT and eNOS activation in LEC and attenuated NO (nitric oxide) production. Further, CD47 silencing in LEC prevented the effects of TSP1 on lymphangiogenic AKT-eNOS signaling and lymphangiogenesis. Atheroprone AAV (adeno-associated virus) 8-PCSK9-injected LEC-specific Cd47 knockout mice (Cd47ΔLEC) had reduced atherosclerosis in both aorta and aortic root compared with control mice (Cd47ΔWT). However, no differences in metabolic parameters including body weight, plasma total cholesterol levels, and fasting blood glucose were observed. Additional immunostaining experiments performed on aortic root cross-sections indicated higher lymphatic vessel density in Cd47ΔLEC mice in comparison to controls.
Conclusions: These findings demonstrate that TSP1 inhibits lymphangiogenesis via activation of CD47 in LEC, and loss of LEC Cd47 attenuates atherosclerotic lesion formation. Collectively, these results identify LEC CD47 as a potential therapeutic target in atherosclerosis.
Keywords: aorta; atherosclerosis; endothelial cells; fasting; lymphangiogenesis.
Conflict of interest statement
Figures

References
-
- Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, et al. . 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2022;145:e4–e17. doi: 10.1161/CIR.0000000000001039 - PubMed
-
- Vuorio T, Nurmi H, Moulton K, Kurkipuro J, Robciuc MR, Ohman M, Heinonen SE, Samaranayake H, Heikura T, Alitalo K, et al. . Lymphatic vessel insufficiency in hypercholesterolemic mice alters lipoprotein levels and promotes atherogenesis. Arterioscler Thromb Vasc Biol. 2014;34:1162–1170. doi: 10.1161/ATVBAHA.114.302528 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

