Disease mutations and phosphorylation alter the allosteric pathways involved in autoinhibition of protein phosphatase 2A
- PMID: 37260014
- PMCID: PMC10238128
- DOI: 10.1063/5.0150272
Disease mutations and phosphorylation alter the allosteric pathways involved in autoinhibition of protein phosphatase 2A
Abstract
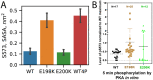
Mutations in protein phosphatase 2A (PP2A) are connected to intellectual disability and cancer. It has been hypothesized that these mutations might disrupt the autoinhibition and phosphorylation-induced activation of PP2A. Since they are located far from both the active and substrate binding sites, it is unclear how they exert their effect. We performed allosteric pathway analysis based on molecular dynamics simulations and combined it with biochemical experiments to investigate the autoinhibition of PP2A. In the wild type (WT), the C-arm of the regulatory subunit B56δ obstructs the active and substrate binding sites exerting a dual autoinhibition effect. We find that the disease mutant, E198K, severely weakens the allosteric pathways that stabilize the C-arm in the WT. Instead, the strongest allosteric pathways in E198K take a different route that promotes exposure of the substrate binding site. To facilitate the allosteric pathway analysis, we introduce a path clustering algorithm for lumping pathways into channels. We reveal remarkable similarities between the allosteric channels of E198K and those in phosphorylation-activated WT, suggesting that the autoinhibition can be alleviated through a conserved mechanism. In contrast, we find that another disease mutant, E200K, which is in spatial proximity of E198, does not repartition the allosteric pathways leading to the substrate binding site; however, it may still induce exposure of the active site. This finding agrees with our biochemical data, allowing us to predict the activity of PP2A with the phosphorylated B56δ and provide insight into how disease mutations in spatial proximity alter the enzymatic activity in surprisingly different mechanisms.
© 2023 Author(s). Published under an exclusive license by AIP Publishing.
Conflict of interest statement
The authors have no conflicts to disclose.
Figures




Similar articles
-
B56δ long-disordered arms form a dynamic PP2A regulation interface coupled with global allostery and Jordan's syndrome mutations.Proc Natl Acad Sci U S A. 2024 Jan 2;121(1):e2310727120. doi: 10.1073/pnas.2310727120. Epub 2023 Dec 27. Proc Natl Acad Sci U S A. 2024. PMID: 38150499 Free PMC article.
-
Extended regulation interface coupled to the allosteric network and disease mutations in the PP2A-B56δ holoenzyme.bioRxiv [Preprint]. 2023 Apr 5:2023.03.09.530109. doi: 10.1101/2023.03.09.530109. bioRxiv. 2023. PMID: 37066309 Free PMC article. Preprint.
-
B56δ-related protein phosphatase 2A dysfunction identified in patients with intellectual disability.J Clin Invest. 2015 Aug 3;125(8):3051-62. doi: 10.1172/JCI79860. Epub 2015 Jul 13. J Clin Invest. 2015. PMID: 26168268 Free PMC article.
-
Protein phosphatase 2A - structure, function and role in neurodevelopmental disorders.J Cell Sci. 2021 Jul 1;134(13):jcs248187. doi: 10.1242/jcs.248187. Epub 2021 Jul 6. J Cell Sci. 2021. PMID: 34228795 Free PMC article. Review.
-
Protein Phosphatase 2A (PP2A) mutations in brain function, development, and neurologic disease.Biochem Soc Trans. 2021 Aug 27;49(4):1567-1588. doi: 10.1042/BST20201313. Biochem Soc Trans. 2021. PMID: 34241636 Review.
Cited by
-
Cardiac myosin binding protein-C phosphorylation as a function of multiple protein kinase and phosphatase activities.Nat Commun. 2024 Jun 14;15(1):5111. doi: 10.1038/s41467-024-49408-5. Nat Commun. 2024. PMID: 38877002 Free PMC article.
-
Non-Markovian Dynamic Models Identify Non-Canonical KRAS-VHL Encounter Complex Conformations for Novel PROTAC Design.JACS Au. 2024 Sep 24;4(10):3857-3868. doi: 10.1021/jacsau.4c00503. eCollection 2024 Oct 28. JACS Au. 2024. PMID: 39483225 Free PMC article.
-
B56δ long-disordered arms form a dynamic PP2A regulation interface coupled with global allostery and Jordan's syndrome mutations.Proc Natl Acad Sci U S A. 2024 Jan 2;121(1):e2310727120. doi: 10.1073/pnas.2310727120. Epub 2023 Dec 27. Proc Natl Acad Sci U S A. 2024. PMID: 38150499 Free PMC article.
-
Extended regulation interface coupled to the allosteric network and disease mutations in the PP2A-B56δ holoenzyme.bioRxiv [Preprint]. 2023 Apr 5:2023.03.09.530109. doi: 10.1101/2023.03.09.530109. bioRxiv. 2023. PMID: 37066309 Free PMC article. Preprint.
References
-
- Van Hoof C., Janssens V., De Baere I., Stark M. J. R., de Winde J. H., Winderickx J., Thevelein J. M., Merlevede W., and Goris J., “The Saccharomyces cerevisiae phosphotyrosyl phosphatase activator proteins are required for a subset of the functions disrupted by protein phosphatase 2A mutations,” Exp. Cell Res. 264(2), 372–387 (2001).10.1006/excr.2000.5144 - DOI - PubMed
-
- Wu C.-G., Chen H., Guo F., Yadav V. K., Mcilwain S. J., Rowse M., Choudhary A., Lin Z., Li Y., Gu T., Zheng A., Xu Q., Lee W., Resch E., Johnson B., Day J., Ge Y., Ong I. M., Burkard M. E., Ivarsson Y., and Xing Y., “PP2A-B′ holoenzyme substrate recognition, regulation and role in cytokinesis,” Cell Discovery 3(1), 17027 (2017).10.1038/celldisc.2017.27 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

