PEG-asparaginase treatment regimens for acute lymphoblastic leukaemia in children: a network meta-analysis
- PMID: 37260073
- PMCID: PMC10230854
- DOI: 10.1002/14651858.CD014570.pub2
PEG-asparaginase treatment regimens for acute lymphoblastic leukaemia in children: a network meta-analysis
Abstract
Background: Asparaginase has played a crucial role in the improvement of survival in children with acute lymphoblastic leukaemia (ALL), which is the commonest cancer among children. Survival rates have steadily increased over decades since the introduction of asparaginase to ALL therapy, and overall survival rates reach 90% with the best contemporary protocols. Currently, polyethylene glycolated native Escherichia coli-derived L-asparaginase (PEG-asparaginase) is the preferred first-line asparaginase preparation. Besides its clinical benefits, PEG-asparaginase is well known for severe toxicities. Agreement on the optimal dose, treatment duration, and frequency of administration has never been reached among clinicians.
Objectives: Primary objective To assess the effect of the number of PEG-asparaginase doses on survival and relapse in children and adolescents with ALL. Secondary objectives To assess the association between the number of doses of PEG-asparaginase and asparaginase-associated toxicities (e.g. hypersensitivity, thromboembolism, pancreatitis and osteonecrosis). To undertake a network meta-analysis at dose-level in order to generate rankings of the number of doses of PEG-asparaginase used in the treatment for ALL, according to their benefits (survival and relapse) and harms (toxicity).
Search methods: We searched CENTRAL, PubMed, Embase, Web of Science databases and three trials registers in November 2021, together with reference checking, citation searching and contact with study authors to identify additional studies.
Selection criteria: We included randomised controlled trials (RCTs) comparing different PEG-asparaginase treatment regimens in children and adolescents (< 18 years of age) with first-line ALL treated with multiagent chemotherapy including PEG-asparaginase.
Data collection and analysis: Using a standardised data collection form, two review authors independently screened and selected studies, extracted data, assessed risk of bias for each outcome using a standardised tool (RoB 2.0) and assessed the certainty of evidence for each outcome using the GRADE approach. Primary outcomes included overall survival, event-free survival and leukaemic relapse. Secondary outcomes included asparaginase-associated toxicities (hypersensitivity, thromboembolism, pancreatitis, sinusoidal obstruction syndrome and osteonecrosis as well as overall asparaginase-associated toxicity). We conducted the review and performed the analyses in accordance with the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions.
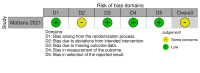
Main results: We included three RCTs in the review, and identified an additional four ongoing studies. We judged outcomes of two RCTs to be at low risk of bias in all the Cochrane risk of bias (RoB 2) domains. We rated the remaining study as having some concerns regarding bias. Due to concerns about imprecision, we rated all outcomes as having low- to moderate-certainty evidence. One study compared intermittent PEG-asparaginase treatment (eight doses of PEG-asparaginase, 1000 IU/m2, intramuscular (IM) administration) versus continuous PEG-asparaginase treatment (15 doses of PEG-asparaginase, 1000 IU/m2, IM) in 625 participants with non-high risk ALL aged 1.0 to 17.9 years. We found that treatment with eight doses probably results in little to no difference in event-free survival compared to treatment with 15 doses (RR 1.01, 95% CI 0.97 to 1.06; moderate-certainty evidence). Compared to treatment with 15 doses, treatment with eight doses may result in either no difference or a slight reduction in hypersensitivity (RR 0.64, 95% CI 0.21 to 1.93; low-certainty evidence), thromboembolism (RR 0.55, 95% CI 0.22 to 1.36; low-certainty evidence) or osteonecrosis (RR 0.68, 95% CI 0.35 to 1.32; low-certainty evidence). Furthermore, we found that treatment with eight doses probably reduces pancreatitis (RR 0.31, 95% CI 0.12 to 0.75; moderate-certainty evidence) and asparaginase-associated toxicity (RR 0.53, 95% CI 0.35 to 0.78; moderate-certainty evidence) compared to treatment with 15 doses. One study compared low-risk standard treatment with additional PEG-asparaginase (six doses, 2500 IU/m2, IM) versus low-risk standard treatment (two doses, 2500 IU/m2, IM) in 1857 participants aged one to nine years old with standard low-risk ALL. We found that, compared to treatment with two doses, treatment with six doses probably results in little to no difference in overall survival (RR 0.99, 95% CI 0.98 to 1.00; moderate-certainty evidence) and event-free survival (RR 1.01, 95% CI 0.99 to 1.04; moderate-certainty evidence), and may result in either no difference or a slight increase in osteonecrosis (RR 1.65, 95% CI 0.91 to 3.00; low-certainty evidence). Furthermore, we found that treatment with six doses probably increases hypersensitivity (RR 12.05, 95% CI 5.27 to 27.58; moderate-certainty evidence), pancreatitis (RR 4.84, 95% CI 2.15 to 10.85; moderate-certainty evidence) and asparaginase-associated toxicity (RR 4.49, 95% CI 3.05 to 6.59; moderate-certainty evidence) compared to treatment with two doses. One trial compared calaspargase (11 doses, 2500 IU/m2, intravenous (IV)) versus PEG-asparaginase (16 doses, 2500 IU/m2, IV) in 239 participants aged one to 21 years with standard- and high-risk ALL and lymphoblastic lymphoma. We found that treatment with 11 doses of calaspargase probably results in little to no difference in event-free survival compared to treatment with 16 doses of PEG-asparaginase (RR 1.06, 95% CI 0.97 to 1.16; moderate-certainty evidence). However, treatment with 11 doses of calaspargase probably reduces leukaemic relapse compared to treatment with 16 doses of PEG-asparaginase (RR 0.32, 95% CI 0.12 to 0.83; moderate-certainty evidence). Furthermore, we found that treatment with 11 doses of calaspargase results in either no difference or a slight reduction in hypersensitivity (RR 1.17, 95% CI 0.64 to 2.13; low-certainty evidence), pancreatitis (RR 0.85, 95% CI 0.47 to 1.52; low-certainty evidence), thromboembolism (RR 0.83, 95% CI 0.48 to 1.42; low-certainty evidence), osteonecrosis (RR 0.63, 95% CI 0.15 to 2.56; low-certainty evidence) and asparaginase-associated toxicity (RR 1.00, 95% CI 0.71 to 1.40; low-certainty evidence) compared to treatment with 16 doses of PEG-asparaginase.
Authors' conclusions: We were not able to conduct a network meta-analysis, and could not draw clear conclusions because it was not possible to rank the interventions. Overall, we found that different numbers of doses of PEG-asparaginase probably result in little to no difference in event-free survival across all studies. In two studies, we found that a higher number of PEG-asparaginase doses probably increases pancreatitis and asparaginase-associated toxicities.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Line Stensig Lynggaard (LSL): None known
Cecilie Utke Rank: None known
Bodil Als‐Nielsen: None known
Sofie Gottschalk Hoejfeldt: None known
Mats Heyman: Chief Investigator for a clinical trial supported by Servier. The support is paid to another institution (Princess Máxima Center, the Netherlands), but the activity will benefit the study. Supported by the Swedish Childhood Cancer Fund, a not‐for‐profit charity, which supports childhood cancer research primarily in Sweden, but also, to some extent, in the Nordic region.
Kjeld Scmiegelow: Unrestricted educational grant from Shire (2018) supporting educational activities on treatment‐related toxicities in childhood ALL. Honorarium from Jazz Pharmaceuticals (2018) as payment for lectures. This author has no conflicts of interest in relation to people with ALL treated with asparaginase.
Birgitte Klug Albertsen: Sponsor for the investigator‐initiated NOR‐GRASPALL 2016 study. Speaker and/or Advisory Board Honoraria from Erytech (2020) and Servier (2021).
Figures




























Update of
- doi: 10.1002/14651858.CD014570
Similar articles
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
-
Taxane monotherapy regimens for the treatment of recurrent epithelial ovarian cancer.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD008766. doi: 10.1002/14651858.CD008766.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866378 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Different corticosteroids and regimens for accelerating fetal lung maturation for babies at risk of preterm birth.Cochrane Database Syst Rev. 2022 Aug 9;8(8):CD006764. doi: 10.1002/14651858.CD006764.pub4. Cochrane Database Syst Rev. 2022. PMID: 35943347 Free PMC article.
-
Treatment options for progression or recurrence of glioblastoma: a network meta-analysis.Cochrane Database Syst Rev. 2021 May 4;5(1):CD013579. doi: 10.1002/14651858.CD013579.pub2. Cochrane Database Syst Rev. 2021. PMID: 34559423 Free PMC article.
Cited by
-
Metabolism in hematology: Technological advances open new perspectives on disease biology and treatment.Hemasphere. 2025 May 19;9(5):e70134. doi: 10.1002/hem3.70134. eCollection 2025 May. Hemasphere. 2025. PMID: 40390870 Free PMC article. Review.
References
References to studies included in this review
Albertsen 2019 {published data only}
-
- Albertsen B K, Abrahamsson J, Lund B, Vettenranta K, Jonsson O G, Frandsen T L, Wolthers B O, Grell K, Heyman M, Schmiegelow K. Intermittent vs continuous asparaginase to reduce asparaginase-associated toxicities: a nopho all2008 randomized study. In: Blood. Vol. 130, sp 1. 2017. - PubMed
-
- Albertsen B K, Grell K, Abrahamsson J, Lund B, Vettenranta K, Jonsson O G, Frandsen T L, Wolthers B O, Heyman M, Schmiegelow K. Intermittent Versus Continuous PEG-Asparaginase to Reduce Asparaginase-Associated Toxicities: A NOPHO ALL2008 Randomized Study. Journal of Clinical Oncology 2019;37(19):1638-1646. - PubMed
-
- Euctr F I. NOPHO ALL2008. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2008-003235-20-FI 2008;(): 2008.
-
- Nct. ALL2008 Protocol for Childhood Acute Lymphoblastic Leukemia Intermittent Versus Continuous PEG Asparaginase. https://clinicaltrials.gov/show/NCT00819351 2009;():2009 2009.
-
- Vogt H, Davidson T, Heyman M, Malmros J, Mogensen N, Stenmarker M, Harila-Saari A. A cost analysis of childhood acute lymphoblastic leukemic randomised to treatment with peg-asparaginase administered at either 2-or 6-week intervals. In: Pediatric Blood & Cancer. Vol. 65. 2018:S109‐.
Mattano 2021 {published data only}
-
- Maloney K W, Devidas M, Wang C, Mattano L A, Friedmann A M, Buckley P, Borowitz M J, Carroll A J, Gastier-Foster J M, Heerema N A, Kadan-Lottick N, Loh M L, Matloub Y H, Marshall D T, Stork L C, Raetz E A, Wood B, Hunger S P, Carroll W L, Winick N J. Outcome in Children With Standard-Risk B-Cell Acute Lymphoblastic Leukemia: Results of Children's Oncology Group Trial AALL0331. Journal of Clinical Oncology 2020;38(6):602-612. - PMC - PubMed
-
- Maloney K, Devidas M, Mattano L, Friedmann A, Rabin K, Buckley P, et al. Excellent Event-Free (EFS) and Overall Survival (OS) for children with Down Syndrome (DS) with Standard Risk B-Acute Lymphoblastic Leukemia (SR B-ALL): results of Children's Oncology Group (COG) AALL0331. In: Pediatric Blood & Cancer. Vol. 65. 2018:S1‐.
-
- Mattano L A, Devidas M, Friedmann A M, Raetz E A, Loh M, Buckley P, Borowitz M J, Carroll A J, Gastier-Foster J, Heerema N A, Kadan-Lottick N, Matloub Y, Marshall D T, Stork L C, Wood B L, Winick N J, Hunger S, Carroll W L, Maloney K. Outstanding outcome for children with standard risk-low (SR-Low) Acute Lymphoblastic Leukemia (ALL) and no benefit to intensified peg-asparaginase (PEG-ASNase) therapy: Results of children's oncology group (COG) Study AALL0331. In: Blood. Conference: 56th Annual Meeting of the American Society of Hematology, ASH. Vol. 124. 2014.
-
- Mattano Leonard A, Devidas Meenakshi, Maloney Kelly W, Wang Cindy, Friedmann Alison M, Buckley Patrick et al. Favorable trisomies and ETV6-RUNX1 predict cure in low-risk B-Cell acute lymphoblastic leukemia: Results from Children's Oncology Group trial AALL0331. Journal of Clinical Oncology 2021;39(14):1540-52. - PMC - PubMed
-
- NCT. Risk-Adapted Chemotherapy in Treating Younger Patients With Newly Diagnosed Standard-Risk Acute Lymphoblastic Leukemia or Localized B-Lineage Lymphoblastic Lymphoma. ClinicalTrials.gov 2010.
Vrooman 2021 {published data only}
-
- SC-PEG Asparaginase vs. Oncaspar in Pediatric Acute Lymphoblastic Leukemia (ALL) and Lymphoblastic Lymphoma. Clinicaltrials.gov. 2012.
-
- Silverman L B, Blonquist T M, Hunt S K, Kay-Green S, Athale U H, Clavell L A, Cole P D, Kelly K M, Laverdiere C, Leclerc J M, et al. Randomized study of pegasparagase (SS-PEG) and calaspargase pegol (SC-PEG) in pediatric patients with newly diagnosed acute lymphoblastic leukemia or lymphoblastic lymphoma: results of DFCI ALL consortium protocol 11-001. In: Blood. Vol. 128. 2016.
-
- Vrooman L M, Blonquist T M, Stevenson K E, Supko J G, Hunt S K, Cronholm S M, Koch V, Kay-Green S, Athale U H, Clavell L A, Cole P D, Harris M H, Kelly K M, Laverdiere C, Leclerc J M, Michon B, Place A E, Schorin M A, Welch J J G, Neuberg D S, Sallan S E, Silverman L B. Efficacy and Toxicity of Pegaspargase and Calaspargase Pegol in Childhood Acute Lymphoblastic Leukemia: Results of DFCI 11-001. Journal of Clinical Oncology 2021;39(31):3496-3505. - PubMed
-
- Vrooman L M, Blonquist T M, Supko J G, Hunt S K, O'Brien J E, Kay-Green S, Athale U H, Clavell L A, Cole P D, Harris M H, et al. Efficacy and toxicity of pegaspargase and calaspargase pegol in childhood acute lymphoblastic leukemia/lymphoma: results of DFCI 11-001. In: Journal of clinical oncology. Vol. 37. 2019. - PubMed
References to studies excluded from this review
Angiolillo 2014 {published data only}
-
- Angiolillo A L, Schore J R, Devidas M, Borowitz J M, Carroll J A. Pharmacokinetic and pharmacodynamic properties of Calaspargase pegol Escherichia coli L-asparaginase in the treatment of patients with acute lymphoblastic leukemia: Results from Children's Oncology Group study AALL07P4. Journal of Clinical Oncology 2014;32(34):3874-82. - PMC - PubMed
-
- Angiolillo A L, Schore J R, Reaman G H, Winick NJ, Devidas M. Pharmacokinetic (PK) and pharmacodynamics (PD) properties of SC-PEG e. coli L-asparaginase (EZN-2285) in the treatment of patients with acute lymphoblastic leukemia(ALL): Results from Children's Oncology Group (COG) study AALL07P4. Journal of Clinical Oncology 2012;30 (15 supplement):9543-9543.
Jeha 2019 {published data only}
-
- Nct. Total Therapy Study XVI for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia. https://clinicaltrials.gov/show/NCT00549848 2007;():2007 2007.
Maloney 2015 {published data only}
-
- Maloney K W, Angiolillo A L, Schore R J, Devidas M, Lu X, Wang C. Association of intravenous (IV) and intramuscular (IM) pegaspargase (PEG) administration with rate of adverse events (AE) in standard risk (SR) acute lymphoblastic leukemia (ALL) Children's Oncology Group (COG) trials. Journal of Clinical Oncology 2015;33(15 (supplement)):10035.
Maloney 2020 {published data only}
Vora 2008 {published data only}
-
- Vora A, Wade R, Mitchell C, Goulden N, Richards S. Efficacy and toxicity of pegylated asparaginase in the treatment of children and young adults with acute lymphoblastic leukaemia: Results of the United Kingdom Medical Research Council (MRC) trial UKALL 2003. Blood 2008;112(11):337-337.
Vora 2010 {published data only}
-
- Vora A J, Mitchell C, Goulden N, Richards S. UKALL 2003, a randomised trial investigating treatment reduction for children and young adults with minimal residual disease defined low risk acute lymphoblastic leukaemia. In: Blood. Vol. 116. 2010. - PubMed
Vora 2013 {published data only}
-
- Vora A, Goulden N, Wade R, Mitchell C, Hancock J, Hough R et al. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncology 2013;14(3):199-209. - PubMed
Vora 2014 {published data only}
-
- Cutting RJ, Richards S, Hancock J, Goulden N, Mitchell CD et al. Post-remission MRD kinetics in children with acute lymphoblastic leukaemia receiving augmented BFM consolidation compared with other regimens: Update of results of the medical research council trial UKALL2003. British Journal of Haematology 2009;145:46-47.
-
- Patrick K, Wade R, Goulden N, Mitchell C, Rowntree C, Hancock J et al. Improved outcome for children and young people with T-acute lymphoblastic leukaemia: Results of the UKALL 2003 trial. In: Blood. Vol. 124. 2014.
-
- Vora A, Goulden N, Mitchell C, Hancock J, Hough R et al. Augmented post-remission therapy for a minimal residual disease-defined high-risk subgroup of children and young people with clinical standard-risk and intermediate-risk acute lymphoblastic leukaemia (UKALL 2003): A randomised controlled trial. The Lancet Oncology 2014;15(8):809-818. - PubMed
References to ongoing studies
NCT 2016 {published data only}
-
- Nct. A French Protocol for the Treatment of Acute Lymphoblastic Leukemia (ALL) in Children and Adolescents. https://clinicaltrials.gov/show/NCT02716233 2016;():2016 2016.
NCT 2021 {published data only}
-
- Nct. A Study Comparing the Blood Levels of Both Pegaspargase (S95014) Formulations (Liquid vs Lyophilized) in the Treatment of Paediatric Patients With Acute Lymphoblastic Leukemia (ALL). https://clinicaltrials.gov/ct2/show/NCT04954326 2021.
NTR 2012 {published data only}
-
- Euctr N L. Treatment with chemotherapy for children >1 and <19 years of age with acute lymphoblastic leukemia. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012-000067-25-NL 2012;(): 2012.
-
- Ntr. Protocol DCOG ALL-11. http://www.who.int/trialsearch/Trial2.aspx?TrialID=NTR3379 2012;(): 2012.
Rizzari 2019 {published data only}
-
- Bleckmann K, Alten J, Schrappe M, Vieth S, Moricke A, Bodmer N, Zapotocka E, Barbaric D, Zimmermann M. Hypoglycemic events during treatment of pediatric acute lymphoblastic leukemia: Observations from trial AIEOP-BFM all 2009. In: Haematologica. Vol. 102. 2017:350-351.
-
- Euctr DE. International collaborative treatment protocol for children and adolescents with acute lymphoblastic leukemia. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2007-004270-43-DE 2009;(): 2009.
-
- Euctr DE. International collaborative treatment protocol for children and adolescents with acute lymphoblastic leukemia. http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2016-001935-12-DE 2018;(): 2018.
-
- Nct. International Collaborative Treatment Protocol For Children And Adolescents With Acute Lymphoblastic Leukemia. https://clinicaltrials.gov/show/NCT01117441 2010;():2010 2010.
-
- Rizzari C, Moericke A, Conter V, Valsecchi M G, Zimmermann M, Silvestri D, Mann G, Niggli F, Dalla-Pozza L, Stary J, Elitzur S, Cario G, Locatelli F, Boos J, Zucchetti M, Biondi A, Schrappe M. Incidence of hypersensitivity reactions (HSR) reactions (HSR) to peg-asparaginase (PEG-ASP) in 6136 patients treated in the AIEOP-BFM ALL 2009 study protocol. In: Blood. Vol. 134. 2019.
Additional references
Ahlke 1997
-
- Ahlke E, Nowak-Gottl U, Schulze-Westhoff P, Werber G, Borste H, Wurthwein G, et al. Dose reduction of asparaginase under pharmacokinetic and pharmacodynamic control during induction therapy in children with acute lymphoblastic leukaemia. British Journal of Haematology 1997;96(4):675-81. - PubMed
Albertsen 2019
-
- Albertsen BK, Grell K, Abrahamsson J, Lund B, Vettenranta K, Jonsson OG, et al. Intermittent versus continuous PEG-asparaginase to reduce asparaginase-associated toxicities: a NOPHO ALL2008 randomized study. Journal of Clinical Oncology 2019;37:Jco1801877. - PubMed
Alexander 2014
-
- Alexander S. Clinically defining and managing high-risk pediatric patients with acute lymphoblastic leukemia. Hematology / the Education Program of the American Society of Hematology 2014;2014(1):181-9. - PubMed
Amylon 1999
-
- Amylon MD, Shuster J, Pullen J, Berard C, Link MP, Wharam M, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia 1999;13(3):335-42. - PubMed
Asselin 1993
-
- Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ. Comparative pharmacokinetic studies of three asparaginase preparations. Journal of Clinical Oncology 1993;11(9):1780-6. - PubMed
Asselin 2015
Atkins 2004
Avramis 2002
-
- Avramis VI, Sencer S, Periclou AP, Sather H, Bostrom BC, Cohen LJ, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children's Cancer Group study. Blood 2002;99(6):1986-94. - PubMed
Avramis 2005
-
- Avramis VI, Panosyan EH. Pharmacokinetic/pharmacodynamic relationships of asparaginase formulations: the past, the present and recommendations for the future. Clinical Pharmacokinetics 2005;44(4):367-93. - PubMed
Beaupin 2017
-
- Beaupin LK, Bostrom B, Barth MJ, Franklin I, Jaeger R, et al. Pegaspargase hypersensitivity reactions: intravenous infusion versus intramuscular injection - a review. Leukemia & Lymphoma 2017;58(4):766-772. - PubMed
Bender 2021
Bohnstedt 2013
-
- Bohnstedt C, Levinsen M, Rosthøj S, Zeller B, Taskinen M, Hafsteinsdottir S, et al. Physicians compliance during maintenance therapy in children with Down syndrome and acute lymphoblastic leukemia. Leukemia 2013;27(4):866-70. - PubMed
Boos 1996
-
- Boos J, Werber G, Ahlke E, Schulze-Westhoff P, Nowak-Gottl U, Wurthwein G, et al. Monitoring of asparaginase activity and asparagine levels in children on different asparaginase preparations. European Journal of Cancer 1996;32a(9):1544-50. - PubMed
Boutron 2021
-
- Boutron I, Page MJ, Higgins JP, Altman DG, Lundh A, Hróbjartsson A, on behalf of the Cochrane Bias Methods Group. Chapter 7: Considering bias and conflicts of interest among the included studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Brigitha 2021
-
- Brigitha LJ, Pieters R, Sluis IM. How much asparaginase is needed for optimal outcome in childhood acute lymphoblastic leukaemia? A systematic review. European Journal of Cancer 2021;157:238-249. - PubMed
Broome 1963
Buitenkamp 2014
Burke 2018
-
- Burke MJ, Devidas M, Maloney K, Angiolillo A, Schore R, Dunsmore K, et al. Severe pegaspargase hypersensitivity reaction rates (grade ≥3) with intravenous infusion vs. intramuscular injection: analysis of 54,280 doses administered to 16,534 patients on children's oncology group (COG) clinical trials. Leukemia & Lymphoma 2018;59(7):1624-1633. - PMC - PubMed
Chaimani 2012
-
- Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Research synthesis methods 2012;3(2):161-76. - PubMed
Chaimani 2017
-
- Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. Journal of Clinical Epidemiology 2017;83:65-74. - PubMed
Chaimani 2019
-
- Chaimani A, Caldwell DM, Li T, Higgins JP, Salanti G. Chapter 11: Undertaking network meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Cooper 2015
Covidence [Computer program]
-
- Covidence. Version accessed 8 November 2022. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Deeks 2019
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Available from www.training.cochrane.org/handbook 2019.
Domenech 2011
-
- Domenech C, Thomas X, Chabaud S, Baruchel A, Gueyffier F, Mazingue F, et al. l-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005-01 randomized trial. British journal of haematology 2011;153(1):58-65. - PubMed
Duval 2002
-
- Duval M, Suciu S, Ferster A, Rialland X, Nelken B, Lutz P, et al. Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer-Children's Leukemia Group phase 3 trial. Blood 2002;99(8):2734-9. - PubMed
Gottschalk 2021
-
- Gottschalk Højfeldt S, Grell K, Abrahamsson J, Lund B, et al. Relapse risk following truncation of pegylated asparaginase in childhood acute lymphoblastic leukemia. Blood 2021;137(17):2373-2382. - PubMed
Graham 2003
-
- Graham M L. Pegaspargase: a review of clinical studies. Advanced Drug Delivery Reviews 2003;55(10):1293-302. - PubMed
Gupta 2020
Guyatt 2008
Haskell 1969
-
- Haskell CM, Canellos GP. l-asparaginase resistance in human leukemia - asparagine synthetase. Biochemical pharmacology 1969;18(10):2578-80. - PubMed
Hempel 2010
-
- Hempel G, Müller H J, Lanvers-Kaminsky C, Würthwein G, Hoppe A, Boos J. A population pharmacokinetic model for pegylated-asparaginase in children. British journal of haematology 2010;148(1):119-25. - PubMed
Henriksen 2014
-
- Henriksen LT, Nersting J, Raja RA, Frandsen TL, Rosthoj S, Schroder H, et al. Cerebrospinal fluid asparagine depletion during pegylated asparaginase therapy in children with acute lymphoblastic leukaemia. British journal of haematology 2014;166(2):213-20. - PubMed
Henriksen 2015
-
- Henriksen LT, Schroder H, Albertsen BK, Harila-Saari A, Heyman M et al. PEG-asparaginase allergy in children with acute lymphoblastic leukemia in the NOPHO ALL2008 protocol. Pediatric Blood and Cancer 2015;62(3):427-433. - PubMed
Henriksen 2017
-
- Henriksen LT, Gottschalk-Hojfeldt S, Schmiegelow K, Frandsen TL, Skov Wehner P et al. Prolonged first-line PEG-asparaginase treatment in pediatric acute lymphoblastic leukemia in the NOPHO ALL2008 protocol-Pharmacokinetics and antibody formation. Pediatric Blood and Cancer 2017;64(12):–. - PubMed
Heo 2019
Higgins 2019a
-
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). The Cochrane Collaboration, 2019. Available from www.cochrane-handbook.org.
Higgins 2019b
-
- Higgins JPT, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2021
-
- Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hunger 2015
-
- Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. The New England journal of medicine 2015;373(16):1541-52. - PubMed
Izraeli 2014
-
- Izraeli S, Vora A, Zwaan C M, Whitlock J. How I treat ALL in Down's syndrome: pathobiology and management. Blood 2014;123(1):35-40. - PubMed
Jackson 2014
Jackson 2019
-
- Jackson RK, Liebich M, Berry P, Errington J, Liu J, Parker C, Moppett J, et al. Impact of dose and duration of therapy on dexamethasone pharmacokinetics in childhood acute lymphoblastic leukaemia-a report from the UKALL 2011 trial. European journal of cancer 2019;120:75-85. - PubMed
Katz 2015
-
- Katz AJ, Chia VM, Schoonen M, Kelsh MA. Acute lymphoblastic leukemia: an assessment of international incidence, survival, and disease burden. Cancer Causes Control 2015;26(11):1627-42. - PubMed
Kawedia 2012
Kawedia 2014
-
- Kawedia J D, Rytting M E. Asparaginase in Acute Lymphoblastic Leukemia. Clinical Lymphoma Myeloma & Leukemia 2014;14:S14-S17. - PubMed
Kloos 2016
-
- Kloos RQ, Pieters R, Escherich G, Sluis IM. Allergic-like reactions to asparaginase: atypical allergies without asparaginase inactivation. Pediatric blood & cancer 2016;63(11):1928-34. - PubMed
Kloos 2019
-
- Kloos R Q H, Pieters R, den Bos C, Eijkelenburg N K A, Jonge R, Sluis I M. The effect of asparaginase therapy on methotrexate toxicity and efficacy in children with acute lymphoblastic leukemia. Leuk Lymphoma 2019;60(12):3002-3010. - PubMed
Kloos 2020
-
- Kloos RQH, Pieters R, Jumelet FMV, Groot-Kruseman HA, den Bos C, Sluis IM. Individualized asparaginase dosing in childhood acute lymphoblastic leukemia. Journal of clinical oncology 2020;38(7):715-724. - PubMed
Kremer 2020
-
- Kremer LCM, Dalen EC. Cochrane Childhood Cancer Group Module .. https://childhoodcancer.cochrane.org/sites/childhoodcancer.cochrane.org/... Update May 2020.
Kwon 2009
Leclercq 2013
-
- Leclercq Edith, Leeflang Mariska M G, Dalen Elvira C, Kremer Leontien C M. Validation of Search Filters for Identifying Pediatric Studies in PubMed. The Journal of Pediatrics 2013;162(3):629-634.e2. - PubMed
Lefebvre 2019
-
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Li 2020
-
- Li RJ, Jin R, Liu C, Cao X, Manning ML, Di XM, et al. FDA approval summary: Calaspargase Pegol-mknl for treatment of acute lymphoblastic leukemia in children and young adults. Clinical cancer research 2020;26(2):328-331. - PubMed
Li 2022
-
- Li T, Higgins JPT, Deeks JJ (editors). Chapter 5: Collecting data. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Liu 2016
Lund 2011
-
- Lund B, Asberg A, Heyman M, Kanerva J, Harila-Saari A, Hasle H, et al. Risk factors for treatment related mortality in childhood acute lymphoblastic leukaemia. Pediatric blood & cancer 2011;56(4):551-9. - PubMed
Lynggaard 2022
McGuinness 2020
-
- McGuinness Luke A, Higgins Julian P T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods 2020;n/a(n/a). - PubMed
Mody 2008
Mogensen 2018
-
- Mogensen SS, Harila-Saari A, Mäkitie O, Myrberg IH, Niinimäki R, Vestli A, et al. Comparing osteonecrosis clinical phenotype, timing, and risk factors in children and young adults treated for acute lymphoblastic leukemia. Pediatric blood & cancer 2018;65(10):e27300. - PubMed
Moghrabi 2007
Moricke 2016
-
- Moricke A, Zimmermann M, Valsecchi M G, Stanulla M, Biondi A, Mann G, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood 2016;127(17):2101-12. - PubMed
Möricke 2008
-
- Möricke A, Reiter A, Zimmermann M, Gadner H, Stanulla M, Dördelmann M, et al. Risk-adjusted therapy of acute lymphoblastic leukemia can decrease treatment burden and improve survival: treatment results of 2169 unselected pediatric and adolescent patients enrolled in the trial ALL-BFM 95. Blood 2008;111(9):4477-89. - PubMed
Müller 2002
-
- Müller HJ, Beier R, da Palma JC, Lanvers C, Ahlke E, Schütz V, et al. PEG-asparaginase (Oncaspar) 2500 U/m(2) BSA in reinduction and relapse treatment in the ALL/NHL-BFM protocols. Cancer chemotherapy and pharmacology 2002;49(2):149-54. - PubMed
Nikolakopoulou 2020
Oparaji 2017
Page 2019
-
- Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis.. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook..
Papakonstantinou 2020
Patrick 2014
-
- Patrick K, Wade R, Goulden N, Rowntree C, Hough R, Moorman AV, et al. Outcome of Down syndrome associated acute lymphoblastic leukaemia treated on a contemporary protocol. British journal of haematology 2014;165(4):552-5. - PubMed
Pennella 2018
-
- Pennella CL, Rossi JG, Baialardo EM, Alonso CN, Guitter MR, Sánchez La Rosa CG, et al. Acute lymphoblastic leukemia in children with Down syndrome: Comparative analysis versus patients without Down syndrome. Archivos argentinos de pediatría 2018;116(4):e500-e507. - PubMed
Pession 2005
-
- Pession A, Valsecchi MG, Masera G, Kamps WA, Magyarosy E, Rizzari C, et al. Long-term results of a randomized trial on extended use of high dose L-asparaginase for standard risk childhood acute lymphoblastic leukemia. Journal of clinical oncology 2005;23(28):7161-7. - PubMed
Pieters 2016
-
- Pieters R, Groot-Kruseman H, Van der Velden V, Fiocco M, den Berg H, Bont E, et al. Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: study ALL10 from the Dutch Childhood Oncology Group. Journal of clinical oncology 2016;34(22):2591-601. - PubMed
Place 2015
-
- Place AE, Stevenson KE, Vrooman LM, Harris MH, Hunt SK, O'Brien JE, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): a randomised, open-label phase 3 trial. The Lancet. Oncology 2015;16(16):1677-90. - PubMed
Prucker 2009
-
- Prucker C, Attarbaschi A, Peters C, Dworzak MN, Potschger U, Urban C, et al. Induction death and treatment-related mortality in first remission of children with acute lymphoblastic leukemia: a population-based analysis of the Austrian Berlin-Frankfurt-Munster study group. Leukemia 2009;23(7):1264-9. - PubMed
Pui 2008
-
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet 2008;371(9617):1030-43. - PubMed
Pui 2013
Rank 2018
Rank 2020
RevMan Web 2019 [Computer program]
-
- Review Manager Web (RevMan Web) [Computer program]. RevMan Web 2019. The Cochrane Collaboration, available at revman.cochrane.org, 2019.
Rizzari 2019
-
- Rizzari C, Moericke A, Conter V, Valsecchi MG, Zimmermann M, Silvestri D, et al. Incidence of hypersensitivity reactions (HSR) to Peg-Asparaginase (PEG-ASP) in 6136 patients treated in the AIEOP-BFM ALL 2009 Study Protocol. Blood 2019;134(Supplement_1):2589-2589.
Schmiegelow 2016
-
- Schmiegelow K, Attarbaschi A, Barzilai S, Escherich G, Frandsen TL, Halsey C, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. The Lancet. Oncology 2016;17(6):e231-9. - PubMed
Schmiegelow 2017
Schünemann 2019a
-
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). 2019;Available from www.training.cochrane.org/handbook.
Schünemann 2019b
-
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook..
Shah 2009
-
- Shah N, Al-Ahmari A, Al-Yamani A, Dupuis L, Stephens D, Hitzler J. Outcome and toxicity of chemotherapy for acute lymphoblastic leukemia in children with Down syndrome. Pediatric blood & cancer 2009;52(1):14-9. - PubMed
Silverman 2001
-
- Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood 2001;97(5):1211-8. - PubMed
Teachey 2013
-
- Teachey DT, Hunger SP. Predicting relapse risk in childhood acute lymphoblastic leukaemia. British journal of haematology 2013;162(5):606-20. - PubMed
Toft 2018
-
- Toft N, Birgens H, Abrahamsson J, Griskevicius L, Hallbook H, Heyman M, et al. Results of NOPHO ALL2008 treatment for patients aged 1-45 years with acute lymphoblastic leukemia. Leukemia 2018;32(3):606-15. - PubMed
van den Berg 2011
-
- den Berg H. Asparaginase revisited. Leukemia & lymphoma 2011;52(2):168-78. - PubMed
van der Sluis 2016
van der Sluis 2018
-
- Sluis IM, Groot-Kruseman H, Te Loo M, Tissing WJ E, den Bos C, Kaspers GJL, et al. Efficacy and safety of recombinant E. coli asparaginase in children with previously untreated acute lymphoblastic leukemia: A randomized multicenter study of the Dutch Childhood Oncology Group. Pediatric blood & cancer 2018;65(8):e27083. - PubMed
Vieira 2002
-
- Vieira Pinheiro JP, Lanversa C, Würthwein G, Beier R, Casimiro da Palma J, Stackelberg A, et al. Drug monitoring of PEG-asparaginase treatment in childhood acute lymphoblastic leukemia and non-Hodgkin's lymphoma. Leukemia & lymphoma 2002;43(10):1911-20. - PubMed
Vrooman 2016
-
- Vrooman LM, Silverman LB. Treatment of Childhood Acute Lymphoblastic Leukemia: Prognostic Factors and Clinical Advances. Current hematologic malignancy reports 2016;11(5):385-94. - PubMed
Vrooman 2018
Whiting 2016
Zeller 2005
-
- Zeller B, Gustafsson G, Forestier E, Abrahamsson J, Clausen N, Heldrup J, et al. Acute leukaemia in children with Down syndrome: a population-based Nordic study. British journal of haematology 2005;128(6):797-804. - PubMed
Zwaan 2002
-
- Zwaan CM, Kaspers GJ, Pieters R, Hählen K, Janka-Schaub GE, Zantwijk CH, et al. Different drug sensitivity profiles of acute myeloid and lymphoblastic leukemia and normal peripheral blood mononuclear cells in children with and without Down syndrome. Blood 2002;99(1):245-51. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

