Interleukin-6 blocking agents for treating COVID-19: a living systematic review
- PMID: 37260086
- PMCID: PMC10237088
- DOI: 10.1002/14651858.CD013881.pub2
Interleukin-6 blocking agents for treating COVID-19: a living systematic review
Abstract
Background: It has been reported that people with COVID-19 and pre-existing autoantibodies against type I interferons are likely to develop an inflammatory cytokine storm responsible for severe respiratory symptoms. Since interleukin 6 (IL-6) is one of the cytokines released during this inflammatory process, IL-6 blocking agents have been used for treating people with severe COVID-19.
Objectives: To update the evidence on the effectiveness and safety of IL-6 blocking agents compared to standard care alone or to a placebo for people with COVID-19.
Search methods: We searched the World Health Organization (WHO) International Clinical Trials Registry Platform, the Living OVerview of Evidence (L·OVE) platform, and the Cochrane COVID-19 Study Register to identify studies on 7 June 2022.
Selection criteria: We included randomized controlled trials (RCTs) evaluating IL-6 blocking agents compared to standard care alone or to placebo for people with COVID-19, regardless of disease severity.
Data collection and analysis: Pairs of researchers independently conducted study selection, extracted data and assessed risk of bias. We assessed the certainty of evidence using the GRADE approach for all critical and important outcomes. In this update we amended our protocol to update the methods used for grading evidence by establishing minimal important differences for the critical outcomes.
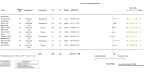
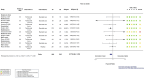
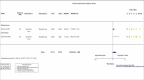
Main results: This update includes 22 additional trials, for a total of 32 trials including 12,160 randomized participants all hospitalized for COVID-19 disease. We identified a further 17 registered RCTs evaluating IL-6 blocking agents without results available as of 7 June 2022. The mean age range varied from 56 to 75 years; 66.2% (8051/12,160) of enrolled participants were men. One-third (11/32) of included trials were placebo-controlled. Twenty-two were published in peer-reviewed journals, three were reported as preprints, two trials had results posted only on registries, and results from five trials were retrieved from another meta-analysis. Eight were funded by pharmaceutical companies. Twenty-six included studies were multicenter trials; four were multinational and 22 took place in single countries. Recruitment of participants occurred between February 2020 and June 2021, with a mean enrollment duration of 21 weeks (range 1 to 54 weeks). Nineteen trials (60%) had a follow-up of 60 days or more. Disease severity ranged from mild to critical disease. The proportion of participants who were intubated at study inclusion also varied from 5% to 95%. Only six trials reported vaccination status; there were no vaccinated participants included in these trials, and 17 trials were conducted before vaccination was rolled out. We assessed a total of six treatments, each compared to placebo or standard care. Twenty trials assessed tocilizumab, nine assessed sarilumab, and two assessed clazakizumab. Only one trial was included for each of the other IL-6 blocking agents (siltuximab, olokizumab, and levilimab). Two trials assessed more than one treatment. Efficacy and safety of tocilizumab and sarilumab compared to standard care or placebo for treating COVID-19 At day (D) 28, tocilizumab and sarilumab probably result in little or no increase in clinical improvement (tocilizumab: risk ratio (RR) 1.05, 95% confidence interval (CI) 1.00 to 1.11; 15 RCTs, 6116 participants; moderate-certainty evidence; sarilumab: RR 0.99, 95% CI 0.94 to 1.05; 7 RCTs, 2425 participants; moderate-certainty evidence). For clinical improvement at ≥ D60, the certainty of evidence is very low for both tocilizumab (RR 1.10, 95% CI 0.81 to 1.48; 1 RCT, 97 participants; very low-certainty evidence) and sarilumab (RR 1.22, 95% CI 0.91 to 1.63; 2 RCTs, 239 participants; very low-certainty evidence). The effect of tocilizumab on the proportion of participants with a WHO Clinical Progression Score (WHO-CPS) of level 7 or above remains uncertain at D28 (RR 0.90, 95% CI 0.72 to 1.12; 13 RCTs, 2117 participants; low-certainty evidence) and that for sarilumab very uncertain (RR 1.10, 95% CI 0.90 to 1.33; 5 RCTs, 886 participants; very low-certainty evidence). Tocilizumab reduces all cause-mortality at D28 compared to standard care/placebo (RR 0.88, 95% CI 0.81 to 0.94; 18 RCTs, 7428 participants; high-certainty evidence). The evidence about the effect of sarilumab on this outcome is very uncertain (RR 1.06, 95% CI 0.86 to 1.30; 9 RCTs, 3305 participants; very low-certainty evidence). The evidence is uncertain for all cause-mortality at ≥ D60 for tocilizumab (RR 0.91, 95% CI 0.80 to 1.04; 9 RCTs, 2775 participants; low-certainty evidence) and very uncertain for sarilumab (RR 0.95, 95% CI 0.84 to 1.07; 6 RCTs, 3379 participants; very low-certainty evidence). Tocilizumab probably results in little to no difference in the risk of adverse events (RR 1.03, 95% CI 0.95 to 1.12; 9 RCTs, 1811 participants; moderate-certainty evidence). The evidence about adverse events for sarilumab is uncertain (RR 1.12, 95% CI 0.97 to 1.28; 4 RCT, 860 participants; low-certainty evidence). The evidence about serious adverse events is very uncertain for tocilizumab (RR 0.93, 95% CI 0.81 to 1.07; 16 RCTs; 2974 participants; very low-certainty evidence) and uncertain for sarilumab (RR 1.09, 95% CI 0.97 to 1.21; 6 RCTs; 2936 participants; low-certainty evidence). Efficacy and safety of clazakizumab, olokizumab, siltuximab and levilimab compared to standard care or placebo for treating COVID-19 The evidence about the effects of clazakizumab, olokizumab, siltuximab, and levilimab comes from only one or two studies for each blocking agent, and is uncertain or very uncertain.
Authors' conclusions: In hospitalized people with COVID-19, results show a beneficial effect of tocilizumab on all-cause mortality in the short term and probably little or no difference in the risk of adverse events compared to standard care alone or placebo. Nevertheless, both tocilizumab and sarilumab probably result in little or no increase in clinical improvement at D28. Evidence for an effect of sarilumab and the other IL-6 blocking agents on critical outcomes is uncertain or very uncertain. Most of the trials included in our review were done before the waves of different variants of concern and before vaccination was rolled out on a large scale. An additional 17 RCTs of IL-6 blocking agents are currently registered with no results yet reported. The number of pending studies and the number of participants planned is low. Consequently, we will not publish further updates of this review.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Lina Ghosn: none known.
Rouba Assi: none known.
Theodoros Evrenoglou: none known.
Brian Buckley: no relevant interests; employed as a systematic reviewer by Cochrane Response during the conduct of the review (with funding from the World Health Organization).
Nicholas Henschke: none known.
Katrin Probyn: none known.
Carolina Riveros: no relevant interests; works as a health professional (epidemiologist).
Mauricia Davidson: none known.
Carolina Graña: none known.
Hillary Bonnet: none known.
Alexander Jarde: none known.
Camila Ávila: none known.
Camilla Hansen Nejstgaard: none known.
Sonia Menon: P95 (consultant).
Gabriel Ferrand: none known.
Philipp Kapp: none known.
Christine Scmucker: none known.
Claudia Breuer: none known
Yanina Sguassero: Cochrane Response (employment); editor of Cochrane Clinical Answers.
Thu Van Nguyen: none known.
Declan Devane: Health Research Board (grant/contract); Registered Midwife but no longer in clinical practice; Editor of Cochrane Pregnancy and Childbirth.
Joerg Meerpohl: none known.
Gabriel Rada: none known.
Asbjørn Hróbjartsson: no relevant interests; Editor of Cochrane Methodology.
Giacomo Grasselli: Pfizer (speaker arrangement).
David Tovey: no relevant interests; Emeritus Editor in Chief and Feedback Editors for two Cochrane Review Groups.
Philippe Ravaud: no relevant interests; involved in Mariette CORIMUNO‐19 Collaborative 2021 (The Ministry of Health, Programme Hospitalier de Recherche Clinique, Foundation for Medical Research, and AP‐HP Foundation).
Anna Chaimani: none known.
Isabelle Boutron: no relevant interests; member of Cochrane Editorial Board.
Figures








































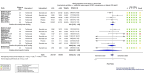





































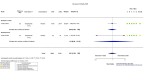


Update of
-
Interleukin-6 blocking agents for treating COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 Mar 18;3(3):CD013881. doi: 10.1002/14651858.CD013881. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Jun 1;6:CD013881. doi: 10.1002/14651858.CD013881.pub2. PMID: 33734435 Free PMC article. Updated.
References
References to studies included in this review
ARCHITECTS 2021 {unpublished data only}
-
- NCT04412772. Trial of tocilizumab for treatment of severe COVID-19: ARCHITECTS (ARCHITECTS). clinicaltrials.gov/ct2/show/NCT04412772 (first received 2 June 2020).
Branch‐Elliman 2022 {published data only (unpublished sought but not used)}
-
- NCT04359901. Sarilumab for patients with moderate COVID-19 disease [Sarilumab for patients with moderate COVID-19 disease: a randomized controlled trial with a play-the-winner design]. clinicaltrials.gov/ct2/show/NCT04359901 (first received 24 April 2020).
Broman 2022 {published data only (unpublished sought but not used)}
-
- Broman N, Feuth T, Vuorinen T, Valtonen M, Hohenthal U, Löyttyniemi E et al. Early administration of tocilizumab in hospitalized COVID-19 patients with elevated inflammatory markers; COVIDSTORM-a prospective, randomized, single-centre, open-label study. Clinical Microbiology and Infection 2022;28(6):844-851. - PMC - PubMed
-
- NCT04577534. Use of tocilizumab in the inflammatory phase of COVID-19 / new coronavirus disease. clinicaltrials.gov/ct2/show/NCT04577534 (first received 8 October 2020).
COVIDOSE‐2 2021 {published and unpublished data}
-
- NCT04479358. Low-dose Tocilizumab versus standard of care in hospitalized patients with COVID-19 (COVIDOSE-2). clinicaltrials.gov/ct2/show/NCT04479358 (first received 21 July 2020).
COVITOZ‐01 2021 {published and unpublished data}
-
- NCT04435717. Efficacy of tocilizumab in modifying the inflammatory parameters of patients with COVID-19 (COVITOZ-01). clinicaltrials.gov/ct2/show/NCT04435717 (first received 17 June 2020).
Declercq 2021 {published data only (unpublished sought but not used)}
-
- NCT04330638. Treatment of COVID-19 patients with anti-interleukin drugs [A prospective, randomized, factorial design, interventional study to compare the safety and efficacy of combinations of blockade of interleukin-6 pathway and interleukin-1 pathway to best standard of care in improving oxygenation and short- and long-term outcome of COVID-19 patients with acute hypoxic respiratory failure and systemic cytokine release syndrome]. clinicaltrials.gov/ct2/show/ (first received 1 April 2020).
Derde 2021 {published data only (unpublished sought but not used)}
-
- Derde LPG, The REMAP-CAP Investigators. Effectiveness of tocilizumab, sarilumab, and anakinra for critically ill patients with COVID-19 The REMAP-CAP COVID-19 Immune Modulation Therapy Domain Randomized Clinical Trial. medRxiv 2021 [Preprint]. [DOI: ]
Garcia‐Vicuna 2022 {published data only (unpublished sought but not used)}
-
- Garcia-Vicuña R, Abad-Santos F, González-Alvaro I, Ramos-Lima F, Sanz JS. Subcutaneous Sarilumab in hospitalised patients with moderate-severe COVID-19 infection compared to the standard of care (SARCOVID): a structured summary of a study protocol for a randomised controlled trial. Trials 2020;21(1):1-4. - PMC - PubMed
-
- NCT04357808. Efficacy of subcutaneous sarilumab in hospitalised patients with moderate-severe COVID-19 infection (SARCOVID) [Randomized open pilot study to evaluate the efficacy of subcutaneous sarilumab in patients with moderate-severe COVID-19 infection]. clinicaltrials.gov/ct2/show/ (first received 22 April 2020).
Gordon 2021 {published data only (unpublished sought but not used)}
-
- NCT02735707. Randomized, embedded, multifactorial adaptive platform trial for community- acquired pneumonia (REMAP-CAP). clinicaltrials.gov/ct2/show/NCT02735707 (first received 13 April 2016).
Hermine 2021 {published and unpublished data}
-
- Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P, et al, CORIMUNO-19 Collaborative Group. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: A randomized clinical trial. JAMA Internal Medicine 2021;181(1):32-40. [DOI: 10.1001/jamainternmed.2020.6820] [PMID: ] - DOI - PMC - PubMed
-
- NCT04331808. CORIMUNO-19 - tocilizumab trial - TOCI (CORIMUNO-TOCI) (CORIMUNO-TOC). clinicaltrials.gov/ct2/show/NCT04331808 (first received 2 April 2020).
Hermine 2022 {published and unpublished data}
-
- Hermine O, Mariette X, Porcher R, Resche-Rigon M, Tharaux PL, Ravaud P, CORIMUNO-19 Collaborative Group. Effect of interleukin-6 receptor antagonists in critically ill adult patients with COVID-19 pneumonia: two randomised controlled trials of the CORIMUNO-19 Collaborative Group. European Respiratory Journal 2022;60(2):2102523. [DOI: 10.1183/13993003.02523-2021] [PMID: ] - DOI - PMC - PubMed
HMO‐0224‐20 2021 {unpublished data only}
-
- HMO-0224-20. The use of tocilizumab in the management of patients who have severe COVID-19 with suspected pulmonary hyperinflammation. clinicaltrials.gov/ct2/show/NCT04377750 (first received 6 May 2020).
-
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, Spiga F, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: A meta-analysis. JAMA 2021;326(6):499-518. [DOI: 10.1001/jama.2021.11330] [PMID: ] - DOI - PMC - PubMed
Horby 2021b {published and unpublished data}
-
- Horby PW, Pessoa-Amorim G, Peto L, Brightling CE, Sarkar R, Thomas K, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv 2021 [Preprint]. [DOI: 10.1101/2021.02.11.21249258] - DOI
-
- ISRCTN50189673. A randomised trial of treatments to prevent death in patients hospitalised with COVID-19 (coronavirus). isrctn.com/ISRCTN50189673 (first received 30 March 2020).
-
- NCT04381936. Randomised evaluation of COVID-19 therapy (RECOVERY). clinicaltrials.gov/ct2/show/NCT04381936 (first received 11 May 2020).
IMMCOVA 2021 {unpublished data only}
-
- EudraCT 2020-001748-24. A multi-center, randomized, open-label study in patients with COVID-19 and respiratory distress not requiring mechanical ventilation, to compare standard-of-care with anakinra and tocilizumab treatment.The Immunomodulation-CoV Assessment (ImmCoVA) study. www.clinicaltrialsregister.eu/ctr-search/trial/2020-001748-24/SE (first received 20 April 2020).
-
- NCT04412291. A study in patients with COVID-19 and respiratory distress not requiring mechanical ventilation, to compare standard-of-care with Anakinra and Tocilizumab treatment the immunomodulation-CoV assessment (ImmCoVA) Study. clinicaltrials.gov/ct2/show/NCT04412291 (first received 2 June 2020).
-
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, Spiga F, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: A meta-analysis. JAMA 2021;326(6):499-518. [DOI: 10.1001/jama.2021.11330] [PMID: ] - DOI - PMC - PubMed
Jordan 2021 {unpublished data only}
-
- Jordan S. Clazakizumab (Anti-Interleukin 6 (IL-6) Monoclonal) compared to placebo for coronavirus disease 2019 (COVID-19) NCT04348500. clinicaltrials.gov/ct2/show/NCT04348500 2021.
Lescure 2021 {published data only (unpublished sought but not used)}
-
- Lescure FX, Honda H, Fowler RA, Lazar JS, Shi G, Wung P, et al. Sarilumab treatment of hospitalised patients with severe or critical COVID-19: a multinational, randomised, adaptive, phase 3, double-blind, placebo-controlled trial. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.02.01.21250769] - DOI
-
- NCT04327388. Sarilumab COVID-19. clinicaltrials.gov/ct2/show/NCT04327388 (first received 31 March 2020).
Lomakin 2021 {published data only (unpublished sought but not used)}
-
- Lomakin NV, Bakirov BA, Protsenko DN, Mazurov VI, Musaev GH, Moiseeva OM, et al. The efficacy and safety of levilimab in severely ill COVID-19 patients not requiring mechanical ventilation: results of a multicenter randomized double-blind placebo-controlled phase III CORONA clinical study. Inflammation Research 2021;70(10-12):1233-1246. - PMC - PubMed
-
- NCT04397562. A clinical trial of the efficacy and safety of levilimab (BCD-089) in patients with severe COVID-19 [A multicenter, randomized, double-blind, placebo-controlled, adaptively designed clinical trial of the efficacy and safety of levilimab (BCD-089) in patients with severe COVID-19]. clinicaltrials.gov/ct2/show/NCT04397562 (first received 21 May 2020).
Lonze 2022 {published data only (unpublished sought but not used)}
-
- Lonze BE, Spiegler P, Wesson RN, Alachkar N, Petkova E, Weldon EP, et al. A randomized double-blinded placebo controlled trial of Clazakizumab for the treatment of COVID-19 pneumonia with hyperinflammation. Critical Care Medicine 2022;50(9):1348-59. [DOI: 10.1097/CCM.0000000000005591] - DOI - PMC - PubMed
-
- NCT04343989. A randomized placebo-controlled safety and dose-finding study for the use of the IL-6 inhibitor clazakizumab in patients with life-threatening COVID-19 Infection. clinicaltrials.gov/ct2/show/ (first received 14 April 2020).
-
- NCT04659772. A study to evaluate clazakizumab in patients with life-threatening COVID-19 infection. clinicaltrials.gov/ct2/show/NCT04659772 (first received 9 December 2020).
Mariette 2021 {published and unpublished data}
Merchante 2021 {published data only (unpublished sought but not used)}
-
- Merchante N, Cárcel S, Garrido-Gracia JC, Trigo-Rodríguez M, Moreno MÁ E, León-López R, et al. Early use of Sarilumab in patients hospitalized with COVID-19 pneumonia and features of systemic inflammation: the SARICOR randomized clinical trial. Antimicrobial Agents and Chemotherapy 2022;66(2):e0210721. - PMC - PubMed
-
- NCT04357860. Clinical trial of sarilumab in adults with COVID-19. clinicaltrials.gov/ct2/show/NCT04357860 (first received 22 April 2020).
Rosas 2022 {published and unpublished data}
-
- NCT04320615. A study to evaluate the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia (COVACTA) [A randomized, double-blind, placebo-controlled, multicenter study to evaluate the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia]. clinicaltrials.gov/ct2/show/NCT04320615 (first received 25 March 2020).
Rutgers 2021 {published data only}
-
- NL8504. Pre-emptive tocilizumab in hypoxic COVID-19 patients, a prospective randomized trial. trialregister.nl/trial/8504 (first received 6 April 2020).
Salama 2020 {published and unpublished data}
-
- NCT04372186. A study to evaluate the efficacy and safety of tocilizumab in hospitalized participants with COVID-19 pneumonia (EMPACTA). clinicaltrials.gov/ct2/show/NCT04372186 (first received May 1 2020).
Salvarani 2020 {published data only (unpublished sought but not used)}
-
- NCT04346355. Efficacy of early administration of tocilizumab in COVID-19 patients. clinicaltrials.gov/ct2/show/NCT04346355 (first received 15 April 2020).
-
- Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al, RCT-TCZ-COVID-19 Study Group. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia. A randomized clinical trial. JAMA Internal Medicine 2021;181(1):24-31. [DOI: 10.1001/jamainternmed.2020.6615] [PMID: ] - DOI - PMC - PubMed
Samsonov 2022 {unpublished data only}
-
- Samsonov M. Study of the efficacy and safety of a single administration of Olokizumab and RPH-104 with standard therapy in patients with severe Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection (COVID-19). clinicaltrials.gov/ct2/show/NCT04380519 (first received 24 January 2022).
Sancho‐Lopez 2021 {published data only (unpublished sought but not used)}
Sivapalasingam 2022 {published data only (unpublished sought but not used)}
-
- NCT04315298. Evaluation of the efficacy and safety of sarilumab in hospitalized patients with COVID-19 [An adaptive phase 2/3, randomized, double-blind, placebo-controlled study assessing efficacy and safety of sarilumab for hospitalized patients with COVID-19]. clinicaltrials.gov/ct2/show/ (first received 19 March 2020).
Soin 2021 {published data only (unpublished sought but not used)}
-
- CTRI/2020/05/025369. A study on treatment of COVID-19 patients with study drug along with standard of care. trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2020/05/025369 (first received 27 May 2020).
-
- Soin AS, Kumar K, Choudhary NS, Sharma P, Mehta Y, Kataria S, et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicentre, randomised, controlled, phase 3 trial. Lancet Respiratory Medicine 2021;9(5):511-21. - PMC - PubMed
Stone 2020 {published data only (unpublished sought but not used)}
-
- NCT04356937. Efficacy of tocilizumab on patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04356937 (first received 22 April 2020).
-
- Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al, BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. New England Journal of Medicine 2020;383(24):2333-44. [DOI: 10.1056/NEJMoa2028836] [PMID: ] - DOI - PMC - PubMed
Talaschian 2021 {published data only (unpublished sought but not used)}
-
- IRCT20081027001411N4. Effect of TOCILIZUMAB (ACTEMRA) on treatment of COVID-19 [Study of tocilizumab effect on treatment and clinical symptoms and laboratory signs of Iranian COVID-19 patients: a clinical trial study].. en.irct.ir/trial/48396 (first received 9 July 2020).
Veiga 2021 {published data only (unpublished sought but not used)}
-
- NCT04403685. Safety and efficacy of tocilizumab in moderate to severe COVID-19 with inflammatory markers (TOCIBRAS). clinicaltrials.gov/ct2/show/NCT04403685 (first received 27 May 2020).
-
- Veiga VC, Prats JA, Farias DL, Rosa RG, Dourado LK, Zampieri FG, et al, Coalition covid-19 Brazil VI Investigators. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ 2021;372:n84. [DOI: 10.1136/bmj.n84] [PMID: ] - DOI - PMC - PubMed
Wang 2021 {published data only (unpublished sought but not used)}
-
- ChiCTR2000029765. A multicenter, randomized controlled trial for the efficacy and safety of tocilizumab in the treatment of new coronavirus pneumonia (COVID-19). chictr.org.cn/showprojen.aspx?proj=49409 (first received 13 February 2020).
-
- Wang D, Fu B, Peng Z, Yang D, Han M, Li M, et al. Tocilizumab ameliorates the hypoxia in COVID-19 moderate patients with bilateral pulmonary lesions: a randomized, controlled, open-label, multicenter trial. Available at SSRN: papers.ssrn.com/sol3/papers.cfm?abstract_id=3667681 [Preprint with The Lancet] 2020. [DOI: 10.2139/ssrn.3667681] - DOI
References to studies excluded from this review
Albuquerque 2021 {published data only}
Rossotti 2020 {published data only}
Smieszek 2021 {published data only}
Tom 2022 {published data only}
Zafar 2021 {published data only}
-
- Zafar A, Khambhati J, Drobni Z, Gongora C, Horick NK, Foulkes A, et al. Effect of tocilizumab on cardiac injury and dysfunction in COVID-19. Journal of the American College of Cardiology 2021;77(18):3028. [DOI: 10.1016/S0735-1097(21)04383-7] - DOI
References to studies awaiting assessment
EUCTR2020‐001275‐32‐DK {unpublished data only}
-
- EUCTR2020-001275-32-DK. Effectiveness of Interleukin-6 receptor inhibitors in the management of patients with severe SARS-CoV-2 pneumonia: An open-Label multicenter sequential randomized controlled trial. clinicaltrialsregister.eu/ctr-search/trial/2020-001275-32/DK (first entered 2020-03-24).
EUCTR2020‐001290‐74‐ES {unpublished data only}
-
- EUCTR2020-001290-74-ES [Efficacy and safety of sarilumab in the early treatment of hospitalized patients with mild-moderate pneumonia and COVID19 infection versus standard of care]. www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:202... (first received 11 April 2020).
EUCTR2020‐001408‐41‐DE {unpublished data only}
-
- EUCTR2020-001408-41-DE. A prospective, randomized, double blinded placebo-controlled trial to evaluate the efficacy and safety of tocilizumab in patients with severe COVID-19 pneumonia. clinicaltrialsregister.eu//ctr-search/trial/2020-001408-41/DE (first entered 2020-04-07).
EUCTR2020‐001770‐30‐BE {unpublished data only}
-
- EUCTR2020-001770-30-BE. COVID 19: Experimental use of tocilizumab (Roactemra®) in severe SARS-CoV-2 related pneumonia.. clinicaltrialsregister.eu//ctr-search/trial/2020-001770-30/BE (first entered 2020-04-15).
NCT04335071 {unpublished data only}
-
- NCT04335071. Tocilizumab in the treatment of Coronavirus induced disease (COVID-19) (CORON-ACT). clinicaltrials.gov/ct2/show/NCT04335071 (first received April 2, 2020).
NCT04381052 {unpublished data only}
-
- NCT04381052. Study for the use of the IL-6 Inhibitor clazakizumab in patients with life-threatening COVID-19 infection. clinicaltrials.gov/ct2/show/NCT04381052 (first received 8 May 2020).
NCT04616586 {unpublished data only}
-
- NCT04616586. Siltuximab in viral ARds. Study (SILVAR). clinicaltrials.gov/ct2/show/NCT04616586 (first received October 29, 2020).
NCT04690920 {unpublished data only}
-
- NCT04690920. Theranostic implication of complementary medicines against interleukin receptors and Gp-130 proteins. clinicaltrials.gov/ct2/show/NCT04690920 (first received 31 December 2020).
References to ongoing studies
ACTRN12620000580976 {unpublished data only}
-
- ACTRN12620000580976. Tocilizumab for the treatment of COVID-19 in intensive care patients: effect on days free of ventilatory support. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379640&isReview... (first received 19 May 2020).
CTRI/2020/12/029793 {unpublished data only}
-
- CTRI/2020/12/029793. Efficacy and safety of tocilizumab in patients with severe COVID-19 pneumonia on steroid therapy: A prospective, randomized, double blind placebo-controlled trial. ctri.nic.in/Clinicaltrials/showallp.php?mid1=50303&EncHid=&userN... (first received 15 December 2020).
EUCTR2020‐001390‐76‐IT {unpublished data only}
-
- EUCTR2020-001390-76-IT. A phase 3, randomized, open-labeled, multi-center study comparing clinical efficacy and safety of intravenous sarilumab plus standard of care compared to standard of care, in the treatment of patients with severe COVID-19 pneumonia. clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-00... (first received 27 April 2020).
EUCTR2020‐001767‐86‐IE {published data only}
-
- EUCTR2020-001767-86-IE. An open-label, multi-centre, randomised trial comparing different doses of single-dose tocilizumab in adults with severe, non-critical, PCR-confirmed COVID-19 infection with evidence of progressive. clinicaltrialsregister.eu/ctr-search/trial/2020-001767-86/IE (first received 15 April 2020).
IRCT20200510047383N1 {unpublished data only}
-
- IRCT20200510047383N1. Evaluation of the effect of Tocilizumab on outcomes of the severe COVID-19 patients. www.irct.ir/trial/48024 (first received 15 May 2020).
IRCT20200525047570N1 {published data only}
-
- IRCT20200525047570N1. A comparative study of the effects of tocilizumab, interferon-gamma and vitamin C on the recovery of critically ill Covid-19 patients and cytokine storm. www.irct.ir/trial/48583 (first received 30 July 2020).
IRCT20201024049134N2 {unpublished data only}
-
- IRCT20201024049134N2. Efficacy of Tocilizumab in Hospitalized Patients with COVID-19: An open-label placebo-controlled clinical study. www.irct.ir/trial/56613 (first received 4 June 2021).
NCT04361552 {unpublished data only}
-
- NCT04361552. Tocilizumab for the treatment of cytokine release syndrome in patients with COVID-19 (SARS-CoV-2 infection). clinicaltrials.gov/ct2/show/NCT04361552 (First received April 22, 2020).
NCT04452474 {published data only}
-
- NCT04452474. Study of the efficacy and safety of a single administration of Olokizumab vs. placebo in addition to standard treatment in patients with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection (COVID-19). clinicaltrials.gov/ct2/show/NCT04452474 (First received June 26, 2020).
NCT04494724 {unpublished data only}
-
- NCT04494724. Clazakizumab vs. placebo - COVID-19 infection. clinicaltrials.gov/ct2/show/NCT04494724 (first received 31 July 2020).
NCT05187793 {published data only}
-
- NCT05187793. Study of efficacy of different treatment regimens of Olokizumab (RESET). clinicaltrials.gov/ct2/show/NCT05187793 (First received December 24, 2021).
Additional references
Attaway 2021
-
- Attaway AH, Scheraga RG, Bhimraj A, Biehl M, Hatipoğlu U. Severe covid-19 pneumonia: pathogenesis and clinical management. BMJ 2021;372:n436. - PubMed
Balduzzi 2019
Bastard 2020
Boppana 2022
-
- Boppana TK, Mittal S, Madan K, Mohan A, Hadda V, Guleria R. Tocilizumab for COVID-19: A systematic review and meta-analysis of randomized controlled trials. Monaldi Archives for Chest Disease 2022;92(4):2136. - PubMed
Boutron 2020a
Cabanac 2021
Campochiaro 2020
Cancer Discov 2021
-
- COVID-19 use causes tocilizumab shortage. Cancer Discovery 2021;11(12):2950. [DOI: ] - PubMed
Cao 2020
-
- Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. Journal of Allergy and Clinical Immunology 2020;146(1):137-46.e3. [DOI: 10.1016/j.jaci.2020.05.019] [PMID: ] - DOI - PMC - PubMed
Caricchio 2020
Chaimani 2018
Chen 2020
CORIMUNO‐19 Collaborative group 2021
Cruciani 2021
-
- Cruciani F, Amato L, De Crescenzo F, Mitrova Z, Saulle R, Vecchi S, Davoli M. [The praise of uncertainty: a systematic living review to evaluate the efficacy and safety of drug treatments for patients with covid-19.]. Recenti Progressi in Medicina 2021;112(3):195-206. - PubMed
Evrenoglou 2021
-
- Evrenoglou T, Boutron I, Chaimani A. metaCOVID: An R-Shiny application for living meta-analyses of COVID-19 trials. medRxiv 2021 [Preprint]. [DOI: ] - PubMed
Galani 2020
Galvan‐Roman 2021
-
- Galván-Román JM, Rodríguez-García SC, Roy-Vallejo E, Marcos-Jiménez A, Sánchez-Alonso S, Fernández-Díaz C, et al, REINMUN-COVID Group. IL-6 serum levels predict severity and response to Tocilizumab in COVID-19: an observational study. Journal of Allergy and Clinical Immunology 2021;147(1):72-80. [DOI: 10.1016/j.jaci.2020.09.018] [PMID: ] - DOI - PMC - PubMed
Ghosn 2021
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 21 February 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guaraldi 2020
Herold 2020
-
- Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, Bergwelt-Baildon M, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. Journal of Allergy and Clinical Immunology 2020;146(1):128-36.e4. [DOI: 10.1016/j.jaci.2020.05.008] [PMID: ] - DOI - PMC - PubMed
Hertanto 2021
Higgins 2021
-
- Higgins, JPT Thomas, J Chandler, J Cumpston, M Li, T Page, MJ Welch, VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook 2021.
Hojyo 2020
Horby 2021a
Juul 2021
Kalil 2021
Kang 2020
Khan 2021
Kimmig 2020
Kirkham 2018
Kumar 2022
Kyriazopoulou 2021
Laguna‐Goya 2020
Lucas 2020
Manson 2020
Mavridis 2015
Mavridis 2018
Mehta 2020
Nejstgaard 2021
-
- Nejstgaard CH, Fabbri A, Hróbjartsson A. Training manual for RoB 2 risk of bias assessment in COVID-19 trials eligible for the COVID-19 living network meta-analysis. Available from zenodo.org/record/4928079. [DOI: 10.5281/zenodo.4928079] - DOI
Oikonomidi 2020
Ouzzani 2016
PAHO 2022
-
- Pan American Health Organization. Ongoing living update of potential COVID-19 therapeutics options: summary of evidence: rapid review. Available at iris.paho.org/handle/10665.2/52719..
Pedersen 2020
Peng 2022
Pierre 2022
Riley 2011
Schünemann 2022
-
- Schünemann HJ, Neumann I, Hultcrantz M, Brignardello-Petersen R, Zeng L, Murad MH, et al. GRADE guidance 35: update on rating imprecision for assessing contextualized certainty of evidence and making decisions. Journal of Clinical Epidemiology 2022;150:225-42. - PubMed
Schünemann 2022b
-
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Scott 2017
Siemieniuk 2020
Solis‐García Del Pozo 2020
-
- Solis-García Del Pozo J, Galindo MF, Nava E, Jordán J. A systematic review on the efficacy and safety of IL-6 modulatory drugs in the treatment of COVID-19 patients. European Review for Medical and Pharmacological Sciences 2020;24(13):7475-84. [DOI: 10.26355/eurrev_202007_21916] [PMID: ] - DOI - PubMed
Sterne 2019
Stone 2017
Stukas 2020
-
- Stukas S, Hoiland RL, Cooper J, Thiara S, Griesdale DE, Thomas AD, et al. The association of inflammatory cytokines in the pulmonary pathophysiology of respiratory failure in critically ill patients with Coronavirus Disease 2019. Critical Care Explorations 2020;2(9):e0203. [DOI: 10.1097/CCE.0000000000000203] [PMID: ] - DOI - PMC - PubMed
Stukas 2022
Tierney 2007
Tleyjeh 2021
Utrero‐Rico 2021
Verma 2021
Viechtbauer 2010
-
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software 2010;36(3):48. [DOI: 10.18637/jss.v036.i03] - DOI
Webb 2020
White 2008
WHO 2020a
-
- World Health Organization. Rolling updates on coronavirus diseases (COVID-19). Available from www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-h... (accessed 24 February 2021).
WHO 2020b
-
- World Health Organization. Clinical management of severe acute respiratory infection when COVID-19 is suspected. Available from who.int/publications/i/item/10665-332299 (accessed 24 February 2021).
WHO REACT Working Group 2021
-
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, et al. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA 2021;326(6):499-518. - PMC - PubMed
WHO Working Group 2020
Worldometers 2022
-
- Coronavirus Symptoms (COVID-19) - Worldometer. Coronavirus Symptoms (COVID-19). www.worldometers.info/coronavirus/coronavirus-symptoms/ (accessed on 19 July 2022).
Yu 2022
References to other published versions of this review
Boutron 2020b
-
- Boutron I, Chaimani A, Devane D, Meerpohl JJ, Rada G, Hróbjartsson A, et al. Interventions for the prevention and treatment of COVID‐19: a living mapping of research and living network meta‐analysis. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD013769. [DOI: 10.1002/14651858.CD013769] - DOI
CRD42020214700
-
- Ghosn L, Boutron I. Interleukin (IL)-6 blocking agents for the treatment of COVID-19. A living systematic review. PROSPERO 2020 CRD42020214700. Available from: www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020214700 (first received 29 October 2020).

