Nsp14 of SARS-CoV-2 inhibits mRNA processing and nuclear export by targeting the nuclear cap-binding complex
- PMID: 37260089
- PMCID: PMC10415132
- DOI: 10.1093/nar/gkad483
Nsp14 of SARS-CoV-2 inhibits mRNA processing and nuclear export by targeting the nuclear cap-binding complex
Abstract
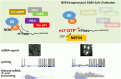
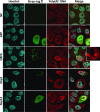
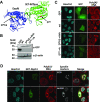
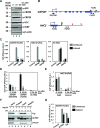
To facilitate selfish replication, viruses halt host gene expression in various ways. The nuclear export of mRNA is one such process targeted by many viruses. SARS-CoV-2, the etiological agent of severe acute respiratory syndrome, also prevents mRNA nuclear export. In this study, Nsp14, a bifunctional viral replicase subunit, was identified as a novel inhibitor of mRNA nuclear export. Nsp14 induces poly(A)+ RNA nuclear accumulation and the dissolution/coalescence of nuclear speckles. Genome-wide gene expression analysis revealed the global dysregulation of splicing and 3'-end processing defects of replication-dependent histone mRNAs by Nsp14. These abnormalities were also observed in SARS-CoV-2-infected cells. A mutation introduced at the guanine-N7-methyltransferase active site of Nsp14 diminished these inhibitory activities. Targeted capillary electrophoresis-mass spectrometry analysis (CE-MS) unveiled the production of N7-methyl-GTP in Nsp14-expressing cells. Association of the nuclear cap-binding complex (NCBC) with the mRNA cap and subsequent recruitment of U1 snRNP and the stem-loop binding protein (SLBP) were impaired by Nsp14. These data suggest that the defects in mRNA processing and export arise from the compromise of NCBC function by N7-methyl-GTP, thus exemplifying a novel viral strategy to block host gene expression.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
The Enzymatic Activity of the nsp14 Exoribonuclease Is Critical for Replication of MERS-CoV and SARS-CoV-2.J Virol. 2020 Nov 9;94(23):e01246-20. doi: 10.1128/JVI.01246-20. Print 2020 Nov 9. J Virol. 2020. PMID: 32938769 Free PMC article.
-
Characterization of the guanine-N7 methyltransferase activity of coronavirus nsp14 on nucleotide GTP.Virus Res. 2013 Sep;176(1-2):45-52. doi: 10.1016/j.virusres.2013.05.001. Epub 2013 May 20. Virus Res. 2013. PMID: 23702198 Free PMC article.
-
Structure-function analysis of the nsp14 N7-guanine methyltransferase reveals an essential role in Betacoronavirus replication.Proc Natl Acad Sci U S A. 2021 Dec 7;118(49):e2108709118. doi: 10.1073/pnas.2108709118. Proc Natl Acad Sci U S A. 2021. PMID: 34845015 Free PMC article.
-
Opportunities and Challenges in Targeting the Proofreading Activity of SARS-CoV-2 Polymerase Complex.Molecules. 2022 May 3;27(9):2918. doi: 10.3390/molecules27092918. Molecules. 2022. PMID: 35566268 Free PMC article. Review.
-
Nuclear functions of the influenza A and B viruses NS1 proteins: do they play a role in viral mRNA export?Vaccine. 2009 Oct 23;27(45):6312-6. doi: 10.1016/j.vaccine.2009.01.015. Vaccine. 2009. PMID: 19840666 Review.
Cited by
-
CBC Complex Regulates Hyphal Growth, Sclerotial Quantity, and Pathogenicity in the Necrotrophic Fungus Botrytis cinerea.J Fungi (Basel). 2025 Jun 2;11(6):429. doi: 10.3390/jof11060429. J Fungi (Basel). 2025. PMID: 40558941 Free PMC article.
-
Development of the coronavirus reverse genetic system: Core technology for pathogenesis mechanisms research and vaccine/drug development.Virulence. 2025 Dec;16(1):2525930. doi: 10.1080/21505594.2025.2525930. Epub 2025 Jun 28. Virulence. 2025. PMID: 40580492 Free PMC article. Review.
-
[Annual review of capillary electrophoresis technology in 2023].Se Pu. 2024 Apr 8;42(5):401-409. doi: 10.3724/SP.J.1123.2024.02007. Se Pu. 2024. PMID: 38736383 Free PMC article. Review. Chinese.
-
The art of hijacking: how Nsp1 impacts host gene expression during coronaviral infections.Biochem Soc Trans. 2024 Feb 28;52(1):481-490. doi: 10.1042/BST20231119. Biochem Soc Trans. 2024. PMID: 38385526 Free PMC article. Review.
-
SARS-CoV-2 and HCoV-OC43 regulate host m6A modification via activation of the mTORC1 signalling pathway to facilitate viral replication.Emerg Microbes Infect. 2025 Dec;14(1):2447620. doi: 10.1080/22221751.2024.2447620. Epub 2025 Feb 24. Emerg Microbes Infect. 2025. PMID: 39745173 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

