Pain Reduction With an Immersive Digital Therapeutic in Women Living With Endometriosis-Related Pelvic Pain: At-Home Self-Administered Randomized Controlled Trial
- PMID: 37260160
- PMCID: PMC10365625
- DOI: 10.2196/47869
Pain Reduction With an Immersive Digital Therapeutic in Women Living With Endometriosis-Related Pelvic Pain: At-Home Self-Administered Randomized Controlled Trial
Abstract
Background: The management of chronic pelvic pain in women with endometriosis is complex and includes the long-term use of opioids. Patients not fully responsive to drugs or ineligible for surgical treatments need efficient alternatives to improve their quality of life and avoid long-term sequelae.
Objective: This randomized controlled trial aimed to assess the effects of repeated at-home administrations of a 20-minute virtual reality (VR) solution (Endocare) compared with a sham condition on pain in women experiencing pelvic pain due to endometriosis.
Methods: Patients were instructed to use the VR headsets twice daily for at least 2 days and for up to 5 days starting on their first day of painful periods. Pain perception was measured using a numerical scale (0-10) before and 60, 120, and 180 minutes after each treatment administration. General pain, stress, fatigue, medication intake, and quality of life were reported daily by patients.
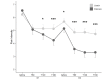
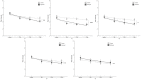
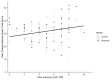
Results: A total of 102 patients with endometriosis were included in the final analysis (Endocare group: n=51, 50%; sham group: n=51, 50%). The mean age was 32.88 years (SD 6.96) and the mean pain intensity before treatment was 6.53 (SD 1.74) and 6.22 (SD 1.69) for the Endocare group and the sham control group, respectively (P=.48). Pain intensity decreased in both groups from day 1 to day 5 along with a decrease in medication use. Maximum pain intensity reduction of 51.58% (SD 35.33) occurred at day 2, 120 minutes after treatment for the Endocare group and of 27.37% (SD 27.23) at day 3, 180 minutes after treatment for the control group. Endocare was significantly superior to the sham on day 1 (120 minutes, P=.04; 180 minutes, P=.001), day 2 (0 minutes, P=.02; 60, 120, and 180 minutes, all P<.001), and day 3 (60 minutes, P=.01; 120 minutes, P=.005; 180 minutes, P=.001). Similarly, the mean perceived pain relief was significantly higher with Endocare on day 1 (120 and 180 minutes P=.004 and P=.001, respectively) and day 2 (60, 120, and 180 minutes P=.003, P=.004, and P=.007, respectively) compared to the control. No adverse event was reported.
Conclusions: This study confirmed the effectiveness and safety of self-repeated administrations of a VR immersive treatment used at home while reducing overall pain medication intake in women diagnosed with endometriosis experiencing moderate-to-severe pelvic pain.
Trial registration: ClinicalTrials.gov NCT05172492; https://clinicaltrials.gov/ct2/show/NCT05172492.
Keywords: chronic pain; digital health; digital health intervention; digital therapeutics; endometriosis; patient outcome; pelvic pain; randomized controlled trial; virtual reality; women's health.
©Benjamin Merlot, Valéry Elie, Adrien Périgord, Zoé Husson, Amandine Jubert, Isabella Chanavaz-Lacheray, Thomas Dennis, Maryne Cotty-Eslous, Horace Roman. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 28.06.2023.
Conflict of interest statement
Conflicts of Interest: VE, AP, and MCE are employees of Lucine company, the developer of the software tested. The other authors have no conflicts to declare.
Figures



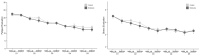
References
-
- Dydyk A, Gupta N. Chronic Pelvic Pain. StatPearls Treasure Island (FL): StatPearls Publishing; 2021. - PubMed
-
- Jia S, Leng J, Shi J, Sun P, Lang J. Health-related quality of life in women with endometriosis: a systematic review. J Ovarian Res. 2012 Oct 18;5(1):29. doi: 10.1186/1757-2215-5-29. https://ovarianresearch.biomedcentral.com/articles/10.1186/1757-2215-5-29 1757-2215-5-29 - DOI - DOI - PMC - PubMed
-
- Saunders PT, Horne AW. Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell. 2021 May 27;184(11):2807–2824. doi: 10.1016/j.cell.2021.04.041. https://linkinghub.elsevier.com/retrieve/pii/S0092-8674(21)00576-6 S0092-8674(21)00576-6 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

