Solution Combustion Synthesis and Characterization of Magnesium Copper Vanadates
- PMID: 37260199
- PMCID: PMC10266371
- DOI: 10.1021/acs.inorgchem.3c00452
Solution Combustion Synthesis and Characterization of Magnesium Copper Vanadates
Abstract
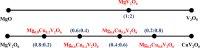
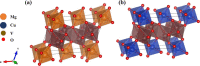
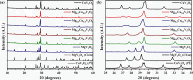
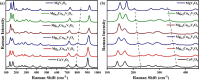
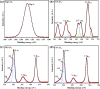
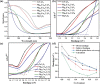
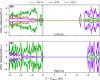
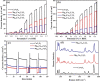
Magnesium vanadate (MgV2O6) and its alloys with copper vanadate were synthesized via the solution combustion technique. Phase purity and solid solution formation were confirmed by a variety of experimental techniques, supported by electronic structure simulations based on density functional theory (DFT). Powder X-ray diffraction combined with Rietveld refinement, laser Raman spectroscopy, diffuse reflectance spectroscopy, and high-resolution transmission electron microscopy showed single-phase alloy formation despite the MgV2O6 and CuV2O6 end members exhibiting monoclinic and triclinic crystal systems, respectively. DFT-calculated optical band gaps showed close agreement in the computed optical bandgaps with experimentally derived values. Surface photovoltage spectroscopy, ambient-pressure photoemission spectroscopy, and Kelvin probe contact potential difference (work function) measurements confirmed a systematic variation in the optical bandgap modification and band alignment as a function of stoichiometry in the alloy composition. These data indicated n-type semiconductor behavior for all the samples which was confirmed by photoelectrochemical measurements.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Hossain M. K.; Sotelo P.; Sarker H. P.; Galante M. T.; Kormányos A.; Longo C.; Macaluso R. T.; Huda M. N.; Janáky C.; Rajeshwar K. Rapid One-Pot Synthesis and Photoelectrochemical Properties of Copper Vanadates. ACS Appl. Energy Mater. 2019, 2, 2837–2847. 10.1021/acsaem.9b00179. - DOI
-
- Hossain M. K.; Sarker H. P.; Sotelo P.; Dang U.; Rodríguez-Gutiérrez I.; Blawat J.; Vali A.; Xie W.; Oskam G.; Huda M. N.; Macaluso R. T.; Rajeshwar K. Phase-Pure Copper Vanadate (α-CuV2O6): Solution Combustion Synthesis and Characterization. Chem. Mater. 2020, 32, 6247–6255. 10.1021/acs.chemmater.0c02227. - DOI
-
- Li P.; Zhou W.; Wang X.; Zhang Y.; Umezawa N.; Abe H.; Ye J.; Wang D. Effects of Cation Concentration on Photocatalytic Performance over Magnesium Vanadates. APL Mater. 2015, 3, 104405. 10.1063/1.4922833. - DOI
LinkOut - more resources
Full Text Sources

