Additional boosting to the RV144 vaccine regimen increased Fc-mediated effector function magnitude but not durability
- PMID: 37260254
- PMCID: PMC10355803
- DOI: 10.1097/QAD.0000000000003611
Additional boosting to the RV144 vaccine regimen increased Fc-mediated effector function magnitude but not durability
Abstract
Objectives: The RV144 vaccine trial resulted in a decreased risk of HIV acquisition that was associated with a nonneutralizing antibody response. The objective of this study was to determine the impact of an additional boost to the RV144 vaccine regimen on antibody effector function and durability.
Design: RV306 was a randomized, double-blind late boosting of the RV144 prime-boost regimen in HIV-uninfected Thai adults (NCT01931358). This analysis included study participants who received the RV144 vaccine regimen and received no additional boost (group 1) or were boosted with ALVAC-HIV and AIDSVAX (group 2) or only AIDSVAX alone (group 3) 24 weeks after completing the RV144 series.
Methods: Plasma samples from RV306 study participants were used to measure antibody-dependent cellular phagocytosis (ADCP), antibody-dependent neutrophil phagocytosis (ADNP), antibody-dependent complement deposition (ADCD), antibody-dependent cellular cytotoxicity (ADCC), trogocystosis, and gp120-specifc IgG subclasses.
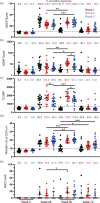
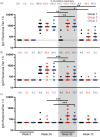
Results: Additional boosting increased the magnitude of all Fc-mediated effector functions 2 weeks following the additional boost compared with 2 weeks after completing the RV144 regimen. However, only trogocytosis remained higher 24-26 weeks after the last vaccination for the study participants receiving an additional boost compared with those that did not receive an additional boost. The additional boost increased IgG1 and IgG4 but decreased IgG3 gp-120 specific antibodies compared with 2 weeks after completing the RV144 regimen.
Conclusion: Additional boosting of RV144 improved the magnitude but not the durability of some Fc-mediated effector functions that were associated with vaccine efficacy, with trogocytosis being the most durable.
Conflict of interest statement
S.G. is an employee and shareholder of Sanofi Pasteur. F.S. is an employee of Global Solutions for Infectious Diseases. All other authors declare no conflicts of interest.
Figures
References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. . MOPH-TAVEG Investigators. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209–2220. - PubMed